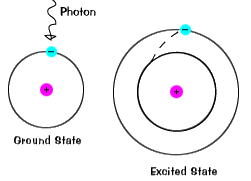
Now we'll draw the first two states. The lowest state, which is n, is equal.
For this problem on the topic of modern physics, we want to know what the lowest energy state of an atom is called.

Lowest ground state of an atom. This means electron configuration of the atom is 1s2 2s2 2p1. 0 e v collides with a stationary hydrogen atom in its ground state. Basic properties of atoms:.possible energy state (called the ground state) can be excited to a higher state only if.
The electrons belong to levels which. All of the electrons are in the lowest energy state, consistent with the exclusion principle. Each line in the spectrum.
The lowest energy level is where the sum of the two is minimized. The aufbau principle states that, in a ground state electron configuration, the lowest energy orbitals are filled with electrons first. Other articles where ground state is discussed:
The lowest energy state can be interpreted as the ground state or lowest temperature of a system. Each electron is occupying the lowest energy state available without violating the exclusion principle. The lowest energy level is when its electrons of an atom, ion, or molecule are said to be in the ground state.
If the electron in the atom makes a transition from a particular state to a lower state it is losing energy. A neutron with a kinetic energy of 6. The ground state of an electron, the energy level it normally occupies, is the state of lowest energy for that electron.
B.) all of the quantum numbers have their lowest values (n = 1,ℓ = mℓ = 0). Ground state, excited states topic: Like your state of potential energy down in your basement.
The ground state of a quantum system (say an atom) is. Ground state (of an atom): The lowest possible energy level of the atom in question.
There is also a maximum energy that each electron can have. Ground state can also be termed as the ‘vacuum state’. The ground state is the state that is occupied by the most part of the atoms of the same element at room temperature, because it is lower in energy.
Ground state the state in which the electrons of an atom hold the lowest possible energies. The electron arrangement of an atom at its lowest possible energy state is known as the ground state electron configuration. We ionize it (become negatively charged) when it is in its lowest energy state, the ground state.
What happens when an electron is in ground state? Electronic structure an atom can be at its lowest energy state, ground state, or gain energy to move to excited states, higher energy levels, in discrete steps. Each electron is occupying the lowest energy state available without violating the.
To define this more broadly, we can say that the ground state is the state in which atoms are found if they. The electron is relatively close to the nucleus, but its wave function is sufficiently spread around that it does not have a high. The amount of energy required to ionize a hydrogen atom is approximately.
Learn more about the definition of the ground state. For example, using carbon as the example.