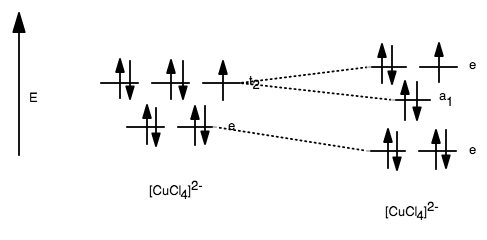
Methane, a common example of a tetrahedral, has a carbon atom surrounded by four hydrogen atoms. An isolated [cucl4] 2− usually has a (meta)stable square planar or flattened tetrahedral structure.
Why [Cucl4]2- Has A Flattened Tetrahedral Structure, Not Regular Tetrahedron And [Cocl4]2- Is Tetrahedron? | Socratic
Synthesis, crystal structure, biomimetic catalysis and dft study
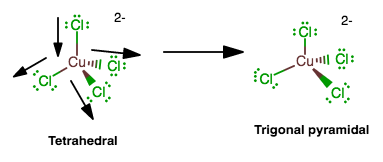
Flattened tetrahedral structure. Please answer me this question. The distribution of ligands in this geometry forms a structure similar to a pyramid, where ligands are located at every corner of the pyramid with one central atom in the middle of the structure. Join / login >> class 10 >> general knowledge >> basic science >> basic chemistry
Likewise, fold 333 is flattened to cause portions of the blank adjacent it to lie in a common plane forming a fourth face of the multiple tetrahedral structure. Request pdf | a rare flattened tetrahedral mn(ii) salen type complex: A rare flattened tetrahedral mn(ii) salen type complex:
See the answer see the answer see the answer done loading. Synthesis, crystal structure, biomimetic catalysis and dft study | a new flattened tetrahedral high spin mn(ii) complex (1. When erected, the structure is.
People tend to get confused between both these molecular geometry because both square planar and tetrahedral has the coordination number of 4. This problem has been solved! The ammonium ion has a central nitrogen atom surrounded by four.
The (cocljion is a regulas tetrahedron but (cuclj has a flattened tetrahedral structure. Weakening of the hydrogen bonding at higher temperature results in the. 10/28/2020 chemistry college answered • expert verified discuss each of the following observations:
For complexes 1b and 2b, the bond lengths around the metal appear to be nearly unaffected by the nature of the ligand contrasted with the difference in the angles (shift from a square planar structure to a flattened tetrahedral one with increasing flexibility of the ligand). In a tetrahedral molecular geometry, a central atom is located at the center with four substituents that are located at the corners of a tetrahedron.the bond angles are cos −1 (− 1 ⁄ 3) = 109.4712206.° ≈ 109.5° when all four substituents are the same, as in methane (ch 4) as well as its heavier analogues.methane and other perfectly symmetrical tetrahedral molecules belong.

Why [Cucl4]2- Has A Flattened Tetrahedral Structure, Not Regular Tetrahedron And [Cocl4]2- Is Tetrahedron? | Socratic