Find the total valence electrons for the h2o molecule. See answer (1) best answer.
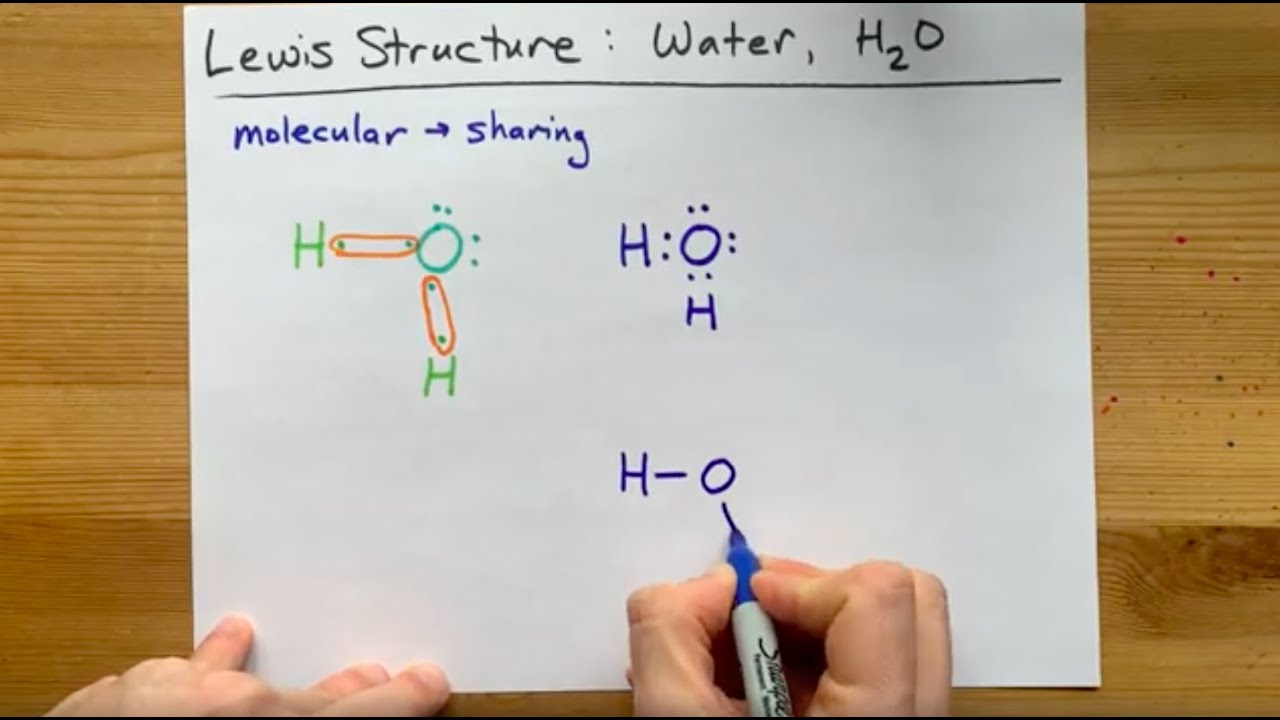
Lewis Structure Of H2O, Water, Dihydrogen Monoxide - Youtube
A) how many groups (atoms and lone pairs) surround the central oxygen?
Lewis structures h20. The lewis structure, proposed by gilbert newton lewis, who introduced it for the first time in 1916, is a graphic representation of the sharing of electrons that occurs in chemical bonds. What is a lewis structure of h2o? The 3d molecular geometry that the vsepr theory predicts for the lewis structure of water would be as follows:
These lone pairs also contribute to the electron repulsion,. O has 6 valence electrons,. The lewis structure of the triatomic h2o molecule shows two single sigma bonds between the oxygen atom and the hydrogen atoms.
Hydrogen = 1 3*hydrogen = 3. A lewis structure is a diagram that shows the bonding between the atoms of a molecule and any possible lone pairs of. Moreover, these bonds leave two lone.
Hydronium ion contains hydrogen and oxygen atoms. The h2o molecule is bent because if you look at the lewis structure you see that the oxygen molecule has two lone pairs. Chemical bond refers to the forces holding atoms together to form.
Lewis structure of water good stuff cheers There are two lone pairs of electrons on each oxygen atom; The lewis structure for water is useful because it allows to determine the molecular geometry and the polarity of the molecule.
Hydrogen sulfide (h2s) is a colorless toxic gas. Draw the lewis structure for water, h20. The lewis structure of the triatomic h2o molecule shows two single sigma bonds between the oxygen atom and the hydrogen atoms.
Around the sulfur atom, there are also two lone. You can find a procedure for drawing lewis structures at this location. Put the least electronegative atom in the.
Because of the two lone pairs, h 2 o will have a bent. There is +1 charge and one lone pair on oxygen atom in h 3 o +. First of all, we need to calculate the total number of valence electrons present in hydronium ion.
In its lewis structure, there are two hydrogen atoms on both sides of the central sulfur atom. Let’s try to draw the lewis structure of h3o+.

Draw A Lewis Structure For H2O That Obeys The Octet Rule If Possible And Answer The Following - Brainly.com