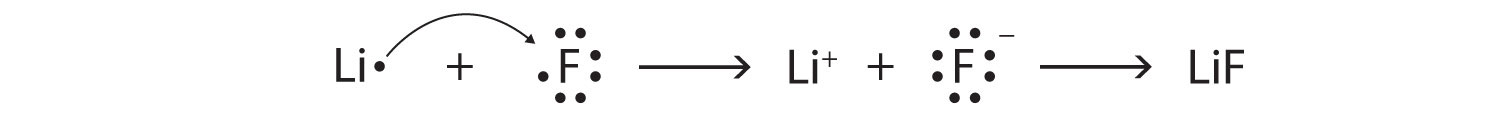However, by doing this, both bonds create elements that are neutrally charged making them stable compounds. An ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally between the atoms.

Ionic Bond: Facts, Definition, Properties, Examples, & Diagrams
The bond formed between any two atoms is not a purely ionic bond.
Is lif an ionic bond. Ionic and covalent bonds are fundamentally different in the way they are formed. An ionic bond is based on attractive electrostatic forces between two ions of opposite charge. After drawing the structures of both and considering dipole moment, doesn't it seems that all k2o should have more ionic character than lif?
Since lithium, the metal has a plus one charge, and fluoride, a nonmetal, has a negative charge, these two ions are held together through an ionic bond. No lif is not an element is lif held together by electrostatic attraction? Mixed bond (not ionic, not covalent).
Jun 14, 2014 lif is lithium fluoride. The greater the lattice energy, the more stable the ionic solid promoted by masterworks Ions are created when an atom loses or gains an electron.
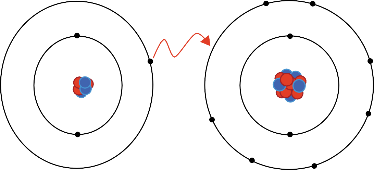
The two main types of chemical bonds are ionic and covalent bonds. This exchange results in a more stable, noble gas electronic configuration for both atoms involved. One shares, and the other trades.
Yes, lithium fluoride has ionic bonds. Does the compound lif have an ionic bond? Lif forms stronger ionic bond than nacl ionic compound melting point lattice energy lif 1017 845 licl 828 610 nacl 788 801 nabr 736 750 mgcl2 2527 714 mgo 3890 2800 cao 3414 2580 1 st value is melting and the next adjacent value is lattice energy.
→ a chemical bond formed when tw…, properties of ionic bonds → high melting and boiling point…, what is a chemical reaction? Real life actions for chemical change? Which ionic compound is predicted to have the greatest bond strength?
Additionally, both bonds focus on the electrons. → chemical change that occurs wh. Ionic vs covalent bonding ionic bondingthe formation of an ionic bond between lithium and fluorine to form lif.
An ionic bond is formed when ions interact to create an ionic compound with the positive and negative charges in balance. An ionic bond is the bond formed by the complete transfer of valence electron to attain stability. Usually, there is some polarity (polar covalent bond.
Cooking an egg, baking a cake. When there is a complete transfer of electrons between the positively charged cation and the negatively charged anion, an electrostatic force of attraction develops, known as the ionic bond. This is an example of a binary ionic compound, which consists of two elements, a cation and anion.
The only pure covalent bonds occur between identical atoms. Real life actions for physical change? 35 terms · what is a covalent bond?
Lif or li2o————————————interview1) revell, k. This exchange of valence electrons allows ions to achieve electronic configurations of the neighbouring noble gases, satisfying the octet rule. Is lif a covalent or a ionic.
My textbook says its lif. The presence of two oppositely charged ions results in a strong attractive force between them. Ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another.
All bonding interactions have some covalent character because the electron density remains shared between the atoms. Is tio2 a ionic or covalent bond? Yes it does is lif an ionic bond?
A binary ionic compound is made of a metal and nonmetal. The electrostatic attraction is between the oppositely charged ions is the ionic bond.