But when a more rigorous approach to mot is applied, it shows that both the bonds present in the molecule must be π bonds and no sigma bonds must be present. Diatomic molecules are incredibly common, particulary in space, so they are of particular interest to physicists, geologists, and astronauts as well.
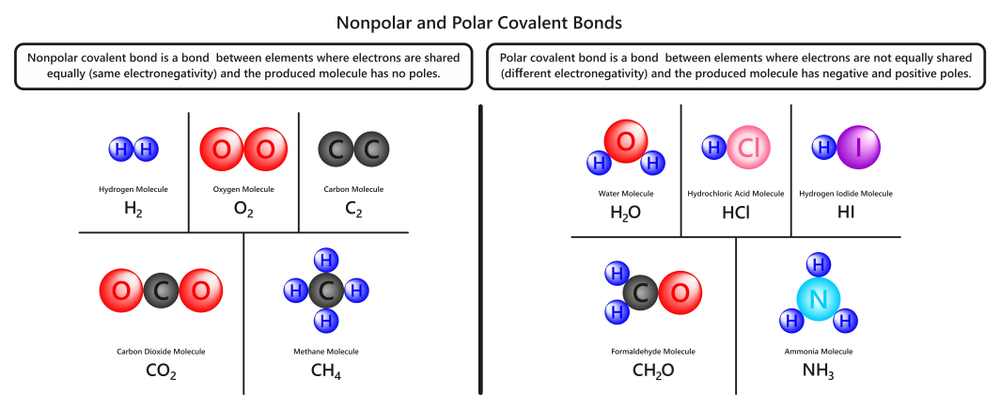
Diatomic Molecules: Definition, Explanation And Examples
Because the number of atoms is 3 in carbon dioxide it is not a diatomic molecule.

Is carbon diatomic. Describe what ways this shape is different from the shape of the σ g orbitals from second period homonuclear diatomic molecules ( see fig. Atomic mass of carbon is 12.0107 u. 8 simple steps weighing the right way recognize and detect the effects of electrostatic charges on your balance chemistry
What is enthalpy sample questions ques. The atoms form a triple bond to become stable and thus become a heteronuclear diatomic molecule. Very special molecules they always exist as a pair of two.
= ( 4 − 0) / 2 = 2 as the bond order is 2, the molecule cannot exist. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. Carbon monoxide is a flammable gas that is odorless, colorless and tasteless.
How do you remember the diatomic elements? As you can see, c isn't one of them. Diatomite is a crucial component of dynamite.
[ 1 ] guide to balance cleaning: There are many deposits of diatomite in north america, both marine and freshwater. Diatomites are commercially mined for many uses.
It is slightly less dense than air. Carbon is not diatomic because it’s not able to form stable* quadruple bonds. (n 2) oxygen (o 2) and carbon monoxide (co).
There are a total of seven diatomic elements. Carbon has 4 and oxygen has 6 valence electrons in the outermost layer. Diatomic carbon is a diatomic molecule of carbon (c 2 ), which occurs when in an electric arc (along with some buckyballs ), in comets, and in the blue light we see in flames.
Carbon dioxide has the chemical formula co2, so contain 1 carbon atom and 2 oxygen atoms. Quadruple bonds, in simple lcao theory, can only form when you can have one bond, and two bonds, and Diatomic elements are molecules composed of two atoms.
Diatomic molecules can be classified into homonuclear and heteronuclear based on the nature of the participating elements. An acronym to remember the diatomic elements is h o br f i n cl. The atomic mass is the mass of an atom.
Hydrogen, carbon and oxygen molecules are some of the common diatomic molecules. Carbon 100% could form a quadruple bond in c2. Define diatomic molecules and give examples?
What are examples of a. Xenon, carbon, and sulfur might form diatomic elements but not under typical room temperature and pressure conditions. Halogens are examples of diatomic molecules.
Diatomic elements are elements that can bond with themselves (the three most common being oxygen, nitrogen, and hydrogen). Examine the shape of the 3 σ orbital of carbon monoxide in figure ( 5.3.2. Diatomaceous earth, or diatomite, is composed by the silica cell walls of diatoms.
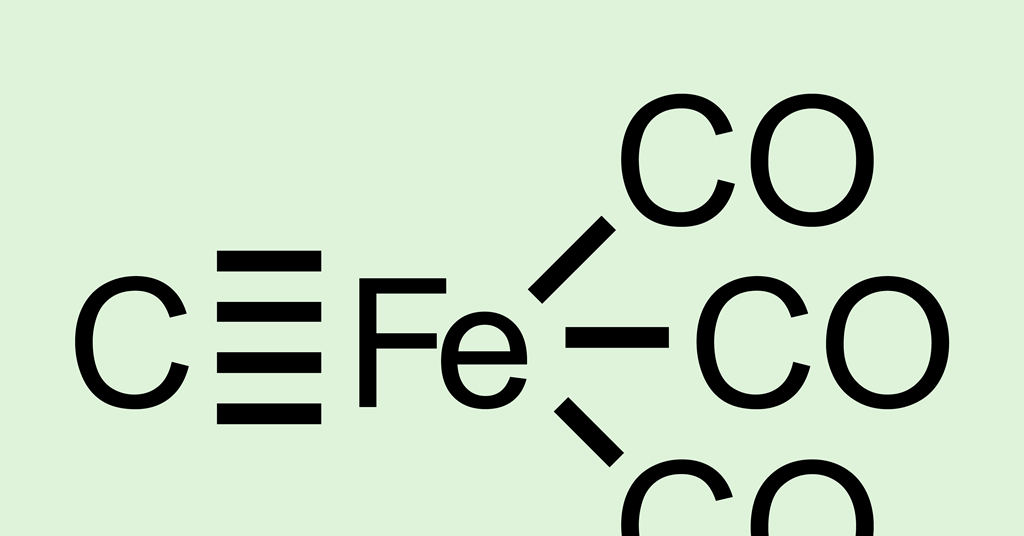
Theoretical Study Predicts Iron–Carbon Quadruple Bond | Research | Chemistry World
