The less dense the gas inside the balloon, the more air it displaces in proportion to its own weight. Air, as in your normal mix of nitrogen and bits of co2 and o2.
However, the net force on the balloon filled with hydrogen would be greater, because helium is more dense than hydrogen.
Helium is more dense. The density of gases depends upon the temperature. But, the pressure in the balloon is pretty tame. Hydrogen is less dense than air.
However the amount of grams per mole is different varying on the gas. Hence why helium balloons float: The result is that warm gases rise and cool gases sink.
It is more than twice as abundant as water vapor (which averages about 4000 ppmv, but varies greatly), 23 times as abundant as carbon dioxide (400 ppmv), and more than 500 times as abundant as neon (18 ppmv). Helium is a biologically inert gas that is less dense than any other known gas except hydrogen and is about one seventh as dense as air. The weight of the helium can cancel out more of the buoyant force than the weight of the hydrogen can.
At the same pressure, helium is less dense than air. The medicinal application of helium and oxygen mixtures (heliox) in the treatment of asthma and extrathoracic airway obstruction has been known for approximately 7 decades. Helium has a molar mass of 4.0026 while hydrogen’s molar mass is 2.0158.
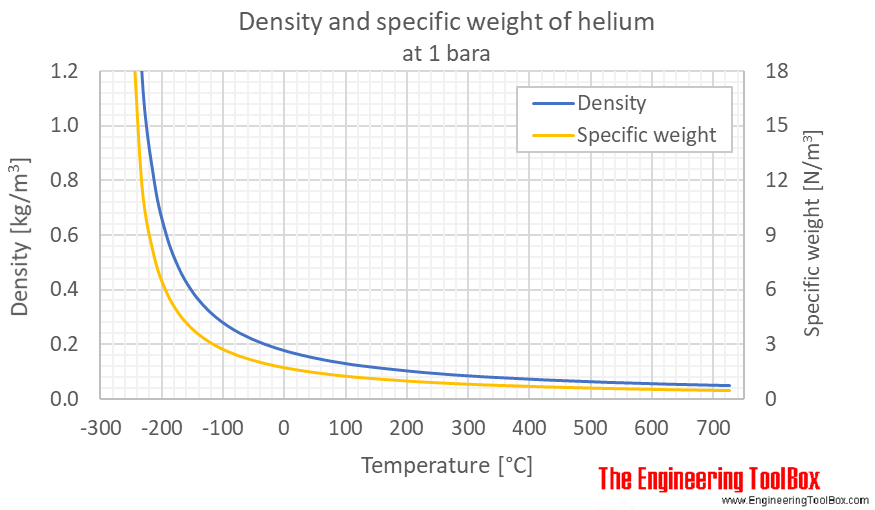
The total mass of the balloon + helium is less than the mass of the volume of air it displaces. Because densi ty equals mass divided by volume, hydrogen is calculated to be less dense than heliu m. The higher the temperature, the more the molecules are spread out and the lower the density as shown in the grapphic on the left.
Gas density examples based upon differences in temperature. Is helium more dense than air? You can infer this without doing any calculations by thinking about the classic example of inhaling the helium from a balloon and talking.
Hydrogen has a mass of 2 grams/ 22.4 liters of volume. ( as well as small percentages of other gases such as hydrogen, carbon dioxide, helium, methane, neon and others) the average. A helium molecule (atom) has a mass of 4 atomic mass units.
At these pressures, helium is much more dense than air, as predicted by the ideal gas law. In their gaseous state, all substances have about the same number of molecules (for monatomic gases like helium, we count atoms as molecules) in a given volume at a given temperature and pressure. The same concept helps to explain the weather.
146 because of its low density, heliox reduces the reynolds. At constant temperature and pressure (stp) the two gases have different weights but constant volume. Air is a mixture of 79 % n_2 nitrogen with a mass of 28 grams/22.4 liters of volume and oxygen with a mass of 32 grams/22.4 liters of volume.
Given that the balloons are filled to the same volume (and the pressure and temperature are the same), the one with helium in it will be heavier; Argon is mostly used as an inert. The pressure in a full tank of helium can reach around 1000x higher than atmospheric.

The Density Of Helium Is Substantially Lower Than Other Gases,... | Download Scientific Diagram
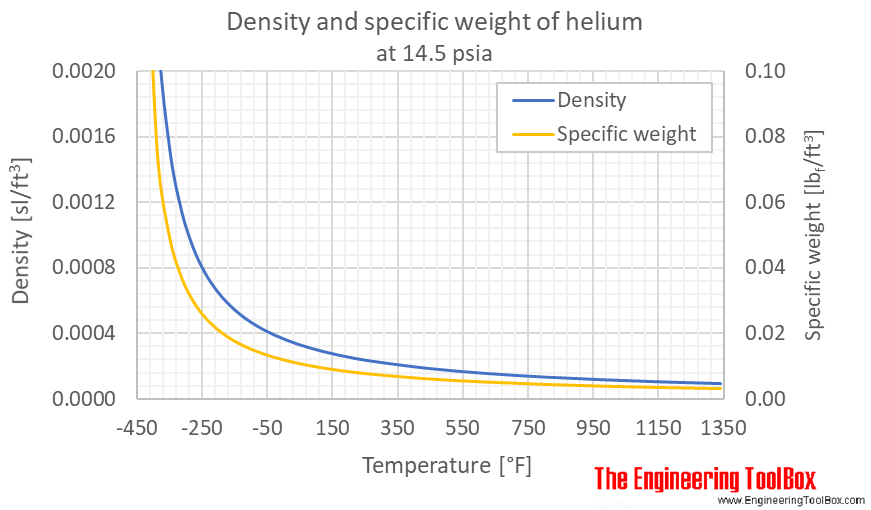
Helium - Density And Specific Weight Vs. Temperature And Pressure
