The n2o molecule has a total. Ozone, or o3 , has two major resonance structures that contribute equally to the overall hybrid structure of the molecule.

N2O Lewis Structure (Dinitrogen Oxide) | Ap Chemistry, Nitrous Oxide, Molecules
The following are the steps to construct the lewis structure.

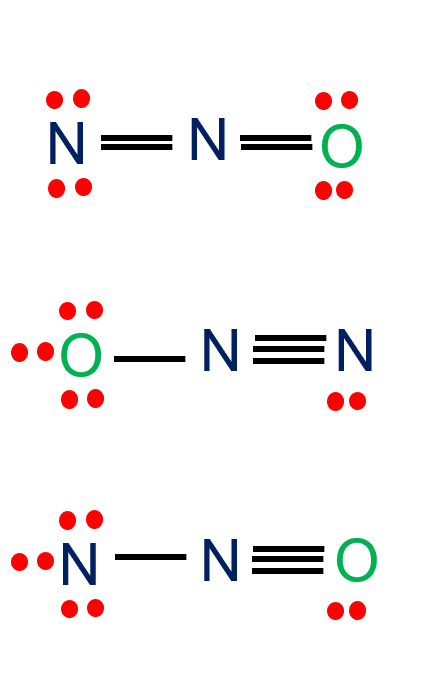
Does n2o have resonance. The central n atom forms bonds with other n atom and o atom as in the. Feb 7, 2015 dinitrogen monooxide, or n2o, has three resonance structures, out of which one is a major contributor and one is a minor contributor. Dinitrogen monooxide, or n2o , has three resonance structures, out of which one is a major contributor and one is a minor contributor.
Does no2+ have equivalent resonance? In n2o, one n atom is located at center and it is attached to another n atom in one side and o atom in other side. @dissenter, no, they are not identical.
N2o or nitrous oxide is commonly known as laughing gas. N2o lewis structure (step by step construction) in the n 2 o lewis structure, the overall ratio of nitrogen to oxygen atom is 2:1. There are several other names by which this compound is known like sweet air, protoxide of nitrogen, etc.
Does n2o have resonance structures? Which nitrogen is which matters for resonance structures in that both can. N2o4 (dinitrogen tetroxide) resonance structures n 2 o 4 resonance structures (dinitrogen dioxide) resonance structures of n2o4 are drawn from the best lewis structure of n 2 o 4.
Does 03 have a resonance structure?
When Determining Lewis Structures, How Do You Know Whether To Use A Double Bond Or A Coordinate Covalent Bond With An Oxygen? - Quora
N2O Lewis Structure| Nitrous Oxide-Laughing Gas - What's Insight