No, metals do not form negative ions: Firstly, we want to write the equation for the reaction that occur to increase the soil ability off aluminum hydroxide.

Why Do Non-Metals Form Negative Ions? - Quora
Do metals form positive or negative ions answered by:

Do mteal teeend o be negaive ions. Researchers looked at 100 years of studies and found evidence. Nonmetals want to gain electrons because they have more valence electrons than metals, so it is easier for them to gain electrons than lose the valance electrons to fulfill a. Nonmetals tend to form negative ions due to the number of valence electrons in their atoms.
Each alkali metal atom has a single. Only metals form positive ions. So in acidic solution, it will be you.
Guangzhou camaz health care co., ltd: To understand some ways in which The ions formed are negative, because they have more electrons than protons.
Do alkali metals form ions? When these metals form ions, the 4s electrons are always lost first,. Only metals form positive ions.
Because they usually are eager to give electron so number of electrons will decrease and number of protons will increase and net charge of the element will be positive. 15 june 2021 tests for ions l. Issued by the chinese ministry:
A 2018 review of ionization literature also found an effect of negative ionization on many facets of human health. Halogens and alkali metals share the common trait of only needed to gain or lose a single electron to form a stable ion. Electrons are those negatively charged particles found orbiting the nucleus of an atom in.
Ions are atoms that can be positively charged (cations) or negatively charged (anions), so cations are formed mostly from metal atoms that tend to lose electrons to reach the nearest noble gas. In the chemistry of the transition elements, the 4s orbital behaves as the outermost, highest energy orbital. And that is they tend to accept electron density, and in doing so (i) become.
No, metals do not form negative ions:

Metal Atoms Lose E- To Form Positive Ions Called Cations: K + Ca 2+ Nh 4 + Nonmetal Atoms Gain E- To Form Negative Ions Called Anions: Cl - S 2- Co Ppt Download
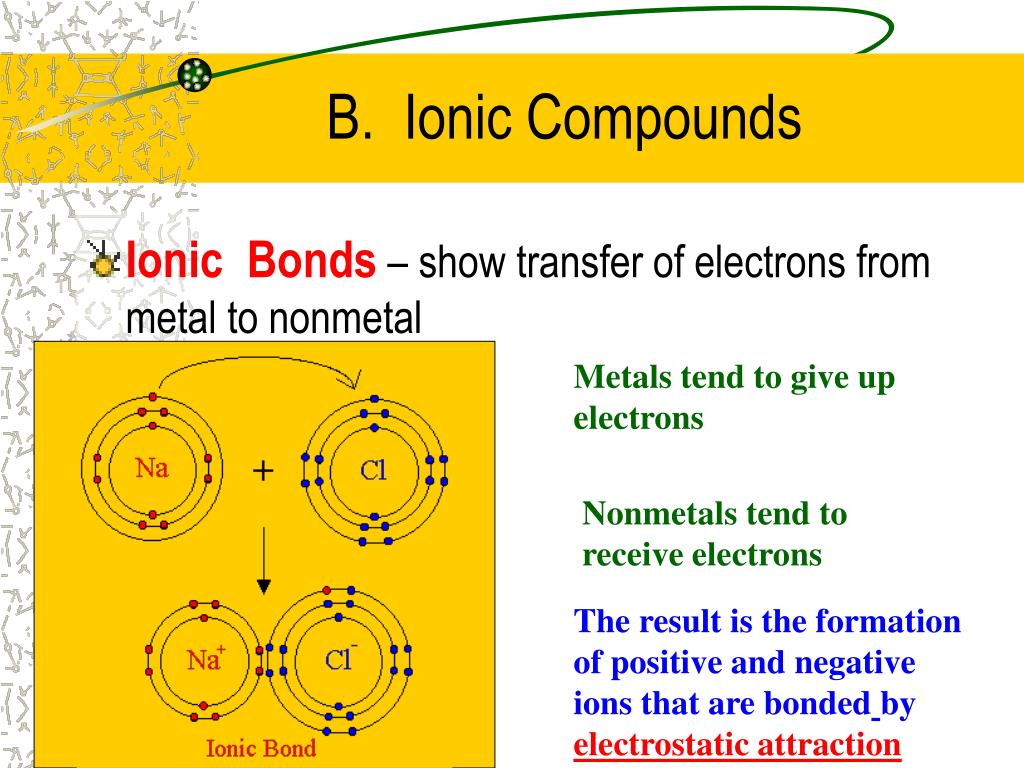
Ppt - Ionic Bonding Powerpoint Presentation, Free Download - Id:6343454