P = pressure v = volume n =. The ideal gas law formula states that pressure multiplied by volume is equal to moles times the universal gas constant times temperature.
Gas Laws Gas Laws Theyll Save Your Life
Boyles law states that “the volume of a given.
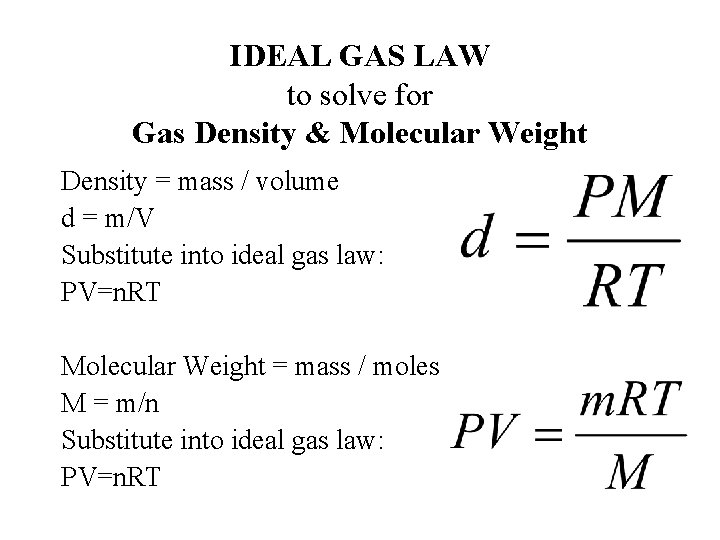
Density ideal gas law formula. Calculating the density of a gas usually involves combining the formula for density (mass divided by volume) and the ideal gas law (pv = nrt). It is a good approximation of real gases under low pressure. The ideal gas law is an equation of state for a gas, which describes the relationships among the four variables temperature (t), pressure (p), volume (v), and moles of gas (n).
Ρ = pm/rt, where m is molar. Empirical/molecular formula problems using the ideal gas law and density of a gas. The volume occupied by one mole of a substance is its molar volume.
According to ideal gas equation, pv = nrt where, p = refers. V = nrt p thus the volume of 1. We can calculate the volume of 1.000 mol of an ideal gas under standard conditions using the variant of the ideal gas law given in equation 6.3.4:
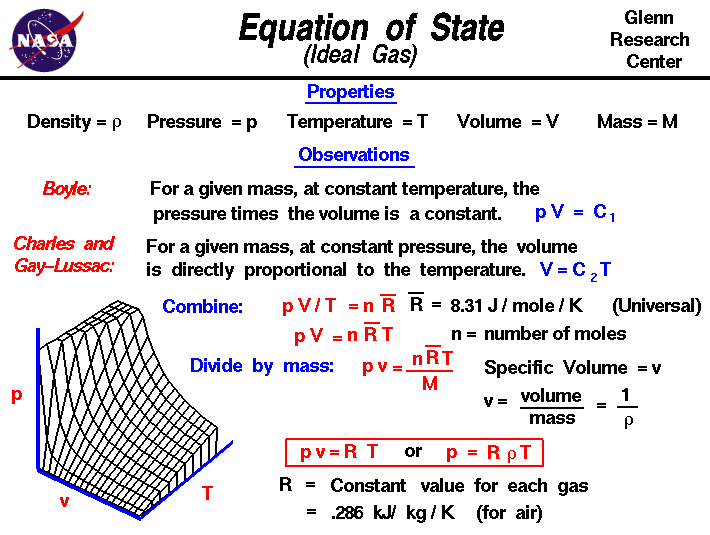
The ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas.it is a good approximation of the behavior of many gases under many conditions,. The ideal gas law, pv = nrt is applicable only ideal gases. The ideal gas law, pv = nrt, suggests that the volume of a given quantity of gas and the number of moles in a given.
Math geometry physics force fluid mechanics finance loan calculator. P is the pressure of the gas, measured in pa;; To calculate the molar mass then consider the ideal gas equation.
Equation of ideal gas law. P v = n r t where: The original ideal gas law uses the formula pv = nrt, the density version of the ideal gas law is pm = drt, where p is pressure measured in atmospheres (atm), t is.
Divide both sides by m: To use this online calculator for density of gas by ideal gas law, enter pressure of gas (p gas), molar mass (m) & temperature of gas (t g) and hit the calculate button. Limitations of ideal gas law the limitations are as follows:
Cyclopropane, a gas once used with oxygen as a general. V is the volume of the gas,. Now you have the ideal gas law rewritten.
We can introduce the density of the gas into the equation by making use of the fact that molecular weight (mw) has units of mass/mole, and thus that n = m/mw, and that the. The original ideal gas law uses the formula pv = nrt, the density version of the ideal gas law is pm = drt, where p is pressure measured in atmospheres (atm), t is temperature. Density of gas is equal to the mass divided by its volume.
Ideal gas law equation calculator solving for density given pressure, specific gas constant and temperature. The density of the gas can be computed using the ideal gas law formula: Density (ρ) is mass per volume.
The state of an ideal gas is determined by the macroscopic and microscopic parameters like pressure, volume, temperature. This equation can be modified to include the density term by the following steps: Thus, the ideal gas equation is.
The ideal gas equation can be derived directly by combining boyle’s law, charles’ law, and avogadro’s law. The properties of an ideal gas are all summarized in one formula of the form:
