Bohr model, description of the structure of atoms, especially that of hydrogen, proposed (1913) by the danish physicist niels bohr. Bohr's model of 1913 for the hydrogen atom.
Problems With Bohr's Model Of The Atom
In these orbits of special radii, the electron does.
Bohr model problems. The bohr atomic model theory made correct predictions for smaller sized atoms like hydrogen, but poor spectral predictions are obtained when larger atoms are considered. A) bohr's model shows the electron circling the nucleus in fixed orbits. This had electrons orbiting a solar nucleus, but involved a techn…
The periodic fable and atomic theory use the following bohr model to answer questions 1 to 6. These failures eventually resulted in abandoning this theory, and the development of the quantum mechanical model of an atom. An example of a flaw demonstrated empirically:
Bohr’s postulates (i) the electrons in an atom revolve around the nucleus in circular orbits without the emission of radiant energy. The emission spectrum of atoms with more than one electron could not be. Equations included in the bohr model of hydrogen atom the difference between the two energy levels (assuming e1, e2) and planck's constant corresponds to the energy absorbed or.
Problems with the bohr model • the bohr model applies only to one electron atoms. Your answer should be numeric and in terms of meters. B) in bohr's model, electrons.
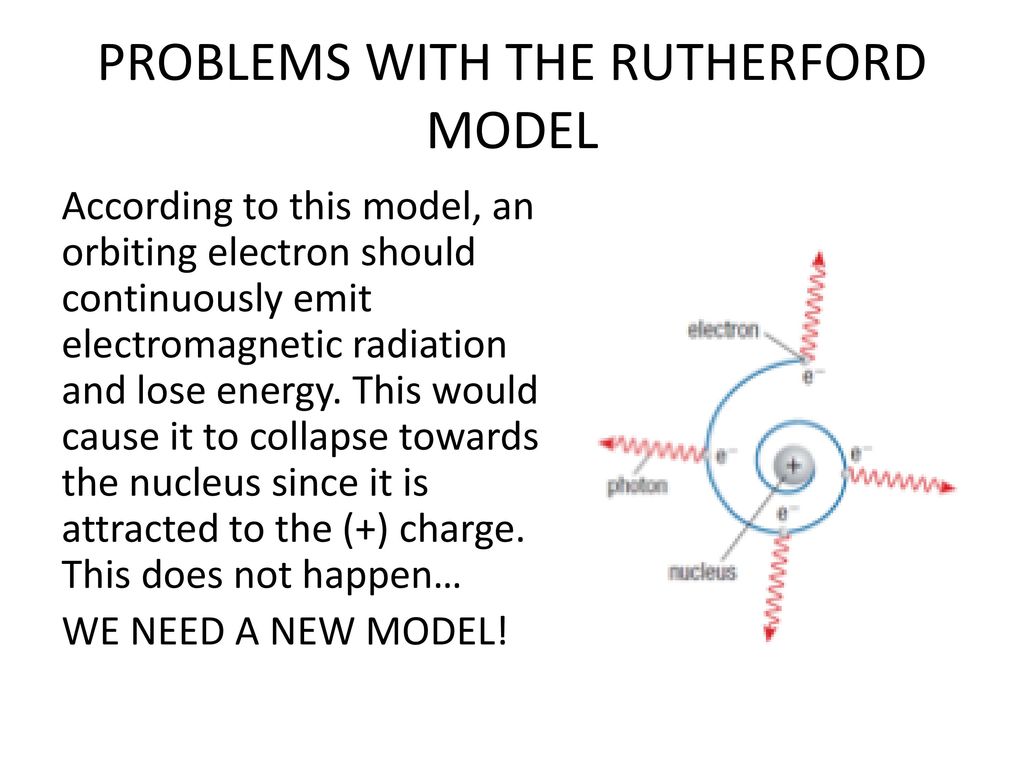
Match the term on the left with the corresponding number on the right. The bohr model predicts the incorrect value for the orbital angular momentum in the ground state of the system. Given this experimental data, rutherford naturally considered a planetary model of the atom, the rutherford model of 1911.
The bohr model met with a lot of problems sadly. The bohr model of the atom, a radical departure from earlier,. Bohr’s model is incomplete, but it nonetheless is helpful in understanding why.
View answer which of the following transitions in a hydrogen atom would emit the. Problems with bohr’s model the bohr atomic model could not accurately describe larger atoms. In 1943, niels bohr described the atom as a planetary system with electrons orbiting around the nucleus.
In the early 20th century, experiments by ernest rutherford established that atoms consisted of a diffuse cloud of negatively charged electrons surrounding a small, dense, positively charged nucleus.

Problems And Solutions On Bohr's Atomic Model | Iit Jee And Neet Physics