Gain of electrons leaves an atom with a net negative charge, and the atom is called an anion. 1 is a graphical depiction of this process.

Why Do Atoms Gain Or Lose Valence Electrons?. To Become Stable Why Do Atoms Gain Or Lose Valence Electrons? - Ppt Download
Does an atom lose electrons?

Why do atoms gain or lose electrons. You mix an acid and water. It is called chemistry of acids. Why do you think atoms lose electrons?
Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the protons in the. Atoms share ,gain, or lose electrons when. An atom will lose or gain electrons to try and fill its outer shell.
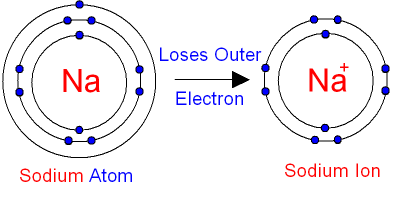
When electrons absorb or release energy their electrons can move to higher or lower energy levels. Atoms and chemical species lose or gain electrons when they. On the left, a sodium atom has 11 electrons.
Ions = charged particles which are formed in ionic bonds. When accepting an electron stabilizes the atom, then the atom will reach a lower energy state by releasing the energy. Yes, atoms (and molecules) can gain or lose protons.
The ionization energy of metals is lower than the ionization energy necessary to take. So atoms or elements, when they exist as ions, they want to lose/gain or share electrons to attain a stable electronic. These ions are atoms that gain or lose electrons, giving them a net positive or negative charge.
Ionic compounds are compounds made up of ions. Why do atoms need to gain or lose electrons? The formation of a sodium ion.
Why do atoms gain or lose electrons when they are ions? This is because, higher the stability, lower is the energy content. Why do atoms gain or lose electrons to form ions?
Elements have to gain/lose electrons because they are automatically attracted to those whom when they connect, they will form a perfect pair of 8 valence electrons, the valence. Why do atoms lose electrons? If an atom has an equal number of protons and electrons its net charge is 0.
A proton is exchanged and you now have a negative ion. If the element is more electronegative, the tendency of it to keep the electrons in it is more. It is easier for iodine to gain an electron rather than to lose 7, so it will form an anion, or negatively charged ion, i−.
Atoms and chemical species lose or gain electrons when they react in order to gain stability. Thus, typically, metals (with nearly empty outer shells) lose. Atoms and chemical species lose or gain electrons when they react in order to gain stability.
Why do atoms gain or lose electrons quizlet? Atoms form ions by losing or gaining electrons because it makes them more stable and this state takes less energy to maintain. The octet rule has been satisfied.
If it loses an electron it. Thus, typically, metals (with nearly. These electrons lose energy by emitting light when they.
If it gains an extra electron it becomes negatively charged and is known as an anion.