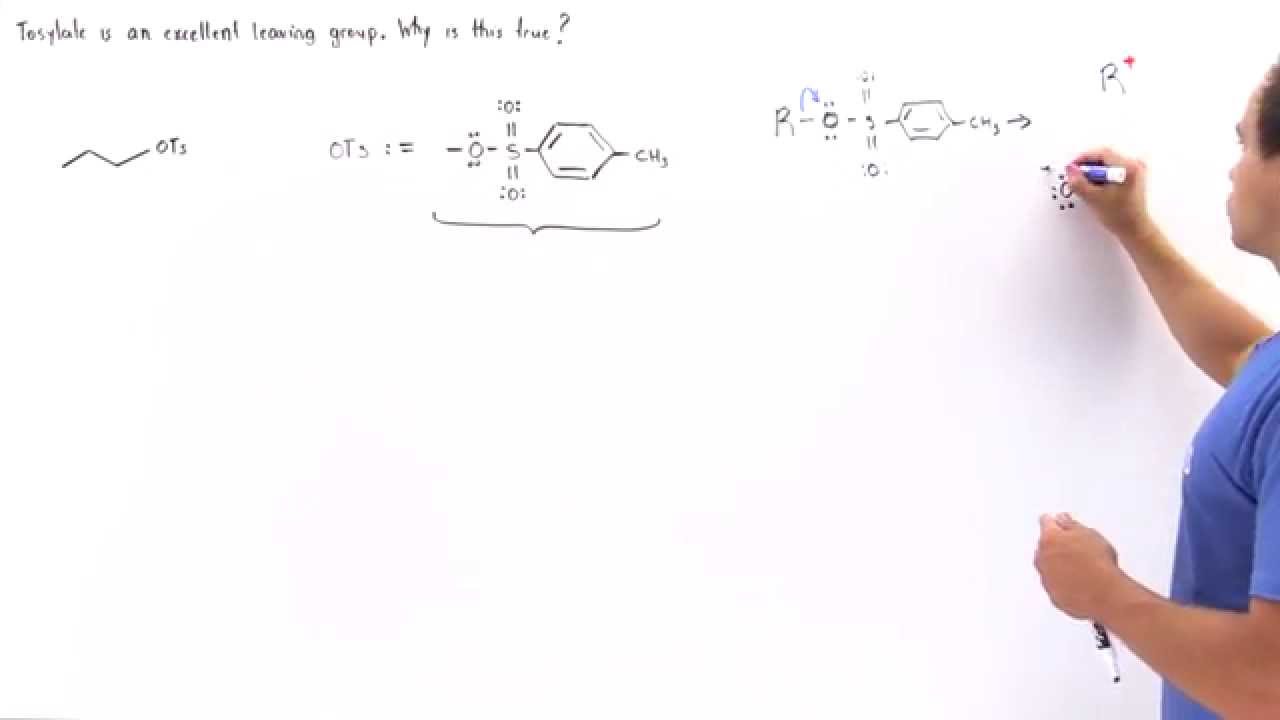After this step, the oh is now turned into a good. Chlorides, bromides, and tosylate / mesylate groups are excellent leaving groups in nucleophilic substitution reactions, due to resonance delocalization of the developing negative charge.

Tosylates And Mesylates – Master Organic Chemistry
Article 258 tfeu enforcement process;
Why are tosylates good leaving groups. Tosylates have a much better leaving group than the original alcohol: Hence, another advantage of using tosylate as protecting group for alcohols is that the alcohol can be reobtained. As we learned previously, resonance stabilized structures are weak bases.
Alkyl sulphates and sulphonates make excellent leaving groups because of this. Tobacco road marathon 2021 results; Resonance increases the ability of the leaving group to leave:
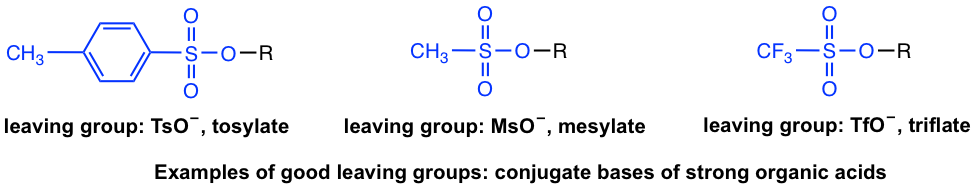
Explain why tosylates make good leaving groups. Tosylate anion also forms stabilized resonance structure on leaving and hence is an excellent leaving group. Triflate, tosylate, and mesylate ions are excellent leaving groups, because the sulfonate ions can stabilize the negative charge via resonance.
Explain why tosylates make good leaving groups. These reactions are favourable for alkyl halides, as halogens are (usually) good leaving groups (halide ions are weak bases). Chlorides, bromides, and tosylate / mesylate groups are excellent leaving groups in nucleophilic substitution reactions, due to resonance delocalization of the developing negative.
$\begingroup$ although i appreciate that brosylates are more prone to solvolytic reactions than tosylates, your link, which reads (remember, brosylate is p. Duncan hines dark chocolate fudge cake mix cookies; Fliers' military branch crossword clue
Alcohols can also react through elimination and. The second arrow always shows a pair of electrons going toward the. The conversion to a sulfonate prevents the alcohol from acting as an acid or nucleophile, or from.
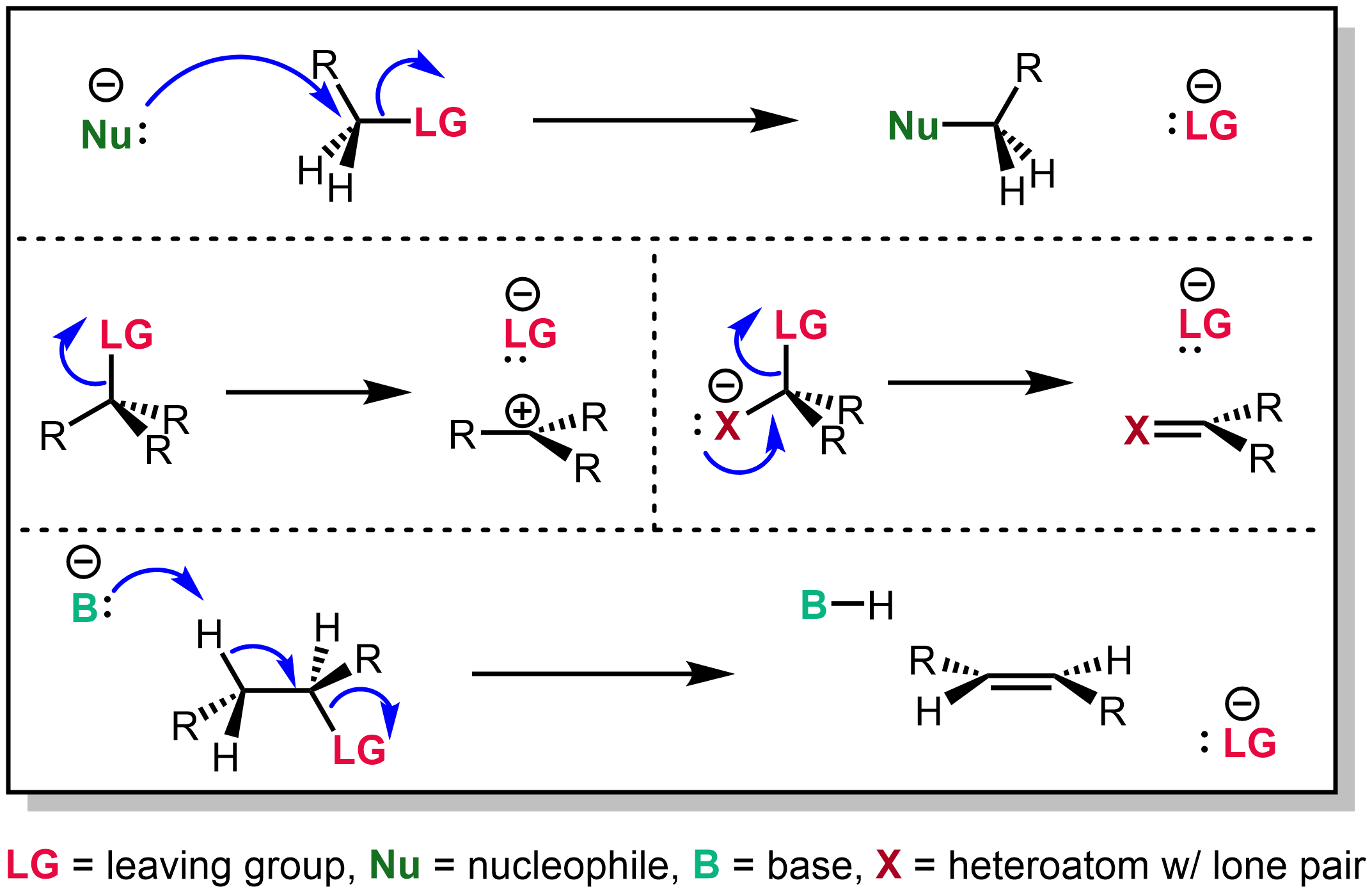
Injecting apple juice in pork The pyridine is added as a base to deprotonate the intermediate and speed up the process of forming the toluenesulfonate ester (tosylate). What is the orientation of the stereogenic center after the reaction depicted in the figure?
Tosylate is a good leaving group. Good leaving groups are weak bases. Csgo dust 2 collection 2021 odds;
Consider a general nucleophilic substitution reaction. What is the orientation of the stereogenic center after the reaction depicted in the figure? Tosylates and mesylates are widely used in the protection of alcohols.
Tosylate groups not only act as.
7.3: Other Factors That Affect Sn2 Reactions - Chemistry Libretexts