Negative ions, by gaining electrons to fill the valence shell b. All atoms strive to have 8 electrons.
How Can We Identify Elements As Metal Or Non-Metals? - Quora
Kq 5 what type of elements ie metals or nonmetals.
What type of charge do nonmetals develop. What charge do group 2a metals form? 1) +2 charge group 2a metals are alkaline earth metals which readily lose 2 electron. Non metals form negatively charged ion.
Hydrogen (sometimes considered an alkali metal) carbon None of the above c. Pages 9 ratings 100% (2) 2 out of 2 people found this document helpful;
Why they are doing is that they need to gain one to three electrons to achieve octet state, making them iso electronic Almost all atoms strive to have 8 protons What is the charge for non metals?
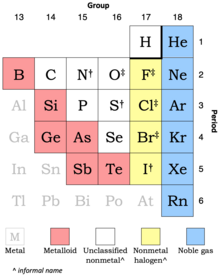
The nonmetals have higher electronegativities than do metals, and compounds formed between metals and nonmetals are generally ionic in nature because of the large differences in electronegativity between them. What charge do group 7a nonmetals form? All atoms strive to have 8 different orbitals.
Group one is composed of metals that have a +1 charge, while all the metals in groups 2,3,4,5,6,7,8,9,10,11,12, and 16 have a charge +2. The metals form cations, the nonmetals form anions, and the resulting compounds are solids under normal conditions. Valance electrons what type of charge do metal ions develop in ionic compounds?
The nonmetals have higher electronegativities than do metals, and compounds formed between metals and nonmetals are generally ionic in nature because of the large differences in electronegativity between them. In normal chemical processes, nonmetals do not form monatomic positive ions (cations) because their ionization energies are too high. By contrast hydrogen, fluorine and iodine only need to gain one electron so they form an anion with a charge of negative one.
What type of elements form anion? The metals form cations, the nonmetals form anions, and the resulting compounds are solids under normal conditions. Positive what does the octet rule say?
When sulphur reacts with oxygen, we get sulphur dioxide. What type of ions do nonmetals form anion negative. These two electrons in the outer shell will be removed to create an ion with a 2 charge to achieve stability and follow the octet rule.
S + o2 → so2 when sulphur dioxide reacts with water it forms sulphurous acid. The nonmetals have higher electronegativities than do metals, and compounds formed between metals and nonmetals are generally ionic in nature because of the large differences in electronegativity between them. List of nonmetals (element group) there are 7 elements that belong to the nonmetals group:
All monatomic nonmetal ions are anions; The metals form cations, the nonmetals form anions, and the resulting compounds are solids under normal conditions. Some common elements that form anions are hydrogen, fluorine, iodine and oxygen.
None of the above a. So2 + h2o → h2so3 5. What type of ions do nonmetals form anion negative circle correct answer 6 are.
Then, metals in groups thirteen and fifteen have a charge of +3. Oxygen needs to gain two electrons so it forms an anion with a charge of negative two. If you look at the periodic table, you will find the metals in groups (from one to 16).
What charge do group 5a nonmetals form? 2 🔴 on a question what type of ions do nonmetals naturally form? Finally, all the metals in group 14 have a +4 charge.

Metals And Non-Metals In The Periodic Table | Philosophical Transactions Of The Royal Society A: Mathematical, Physical And Engineering Sciences