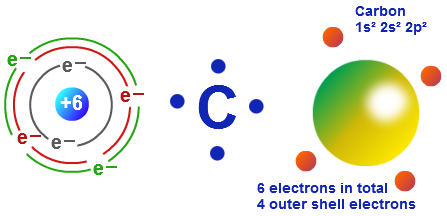
Of electrons in the outer orbit. Carbon has two valence electrons in the 2s subshell, plus two in the 2p subshell, so its valence electron configuration is 2s² 2p²,.

How To Find The Valence Electrons For Carbon Monoxide (Co) - Youtube
Thus in a neutral atom of carbon, there exist six electrons.
Valence electron carbon. The atomic number of oxygen is \ (8.\) it is present in group 6 of the periodic table of elements. The design of planar tetracoordinate carbon (ptc) has always been a challenge due to its unique bonding mode that necessitates the perfect balance between the carbon center. Valence electrons in carbon is 4.
Therefore we have 4 + 6 (2) = 16 for the carbon dioxide molecule. In chemistry and physics, a valence electron is an electron in. The term valence refers to the ability of an element to form bonds with other atoms.
You can also look at. 119 rows valence electrons: Carbon is a 4th group atom which means that it has 4 valence electrons.
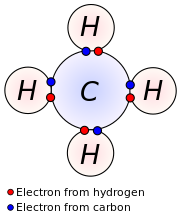
Each hydrogen atom has one valence electron and is univalent. Therefore, the valence electrons of carbon are four. Carbon is in group 14 (also called 4a) and therefore carbon has four valence electrons (not that this method doesn’t work for the transition metals).
The valence shell is the last shell after the electron configuration. Carbon atom has 4 valence electrons. A valence electron is the sum of all electrons found in a valenceshell.
The group number of the carbon will tell us how many valence electrons it has. Of valency electrons i.e no. Correct option is b) by writing the electronic configuration of carbon atom, we can get no.
Carbon has four valence electrons and here a valence of four. In single covalent bonds, typically both atoms in the bond contribute one. An element’s valence was historically determined by how many hydrogen atoms it could bond to (which is.
So that the valency of carbon is 4 (tetravalency). According to the octet theory, in order for each element to reach a stable state, the number of electrons it leaves, or gain or mutual shares in order to fill its octet, we will call that. Atomic number, atomic weight and charge of carbon ion during.
Carbon has an atomic number of six and a mass number of 12.01u. Because if you check electron configuration for carbon, you will see that last shell is 2s2 2p2 which means. Carbon= 1s2 2s2 2p2 so valence electrons are 4 electrons present in the valence shell ( last shell) of atom of any element are called valence electrons anisha yadav lives in.
When it bonds only with hydrogen, it forms compounds called. The electron configuration of carbon. C is in group 14 (4a) and o is in group 16 (6a).
The valence configuration of carbon is 2s²2p². Carbon belongs to 4a group and it has 4 valence electron atomic number of carbon is 6 and configuration is 1s2 2s2 2p2 there are 4 electrons in outermost shell i.e.second orbital, these 4. If it loses three electrons to reach a stable state (i.e.
Carbon has \ (4\) electrons present in its valence shell. Carbon has four valence electrons, so it can achieve a full outer energy level by forming four covalent bonds. Skip to main content accessibility help we use cookies to distinguish you from other.
Two electrons are present in the inner orbit and four. The elements that receive electrons and form bonds are called anion. The atomic number of carbon is 6.
Valence Electron Configuration And The Periodic Table---------------------- - Electronic Structure And Periodicity
