Paramagnetic with four unpaired electrons. How many valence electrons does sn 2+ have?.

Organic Chemistry - The Effect Of The Groups At Para Position On Sn2 Reactivity - Chemistry Stack Exchange
The concept of inert pair effect is being neglected now a days.

Sn+2 electrons. So its valence shell has ten 4d, two 5s, and two 5p electrons. What ion has 50 protons and 48 electrons? Where e∘ ox = −e∘ red is the standard oxidation potential.
Eventually, the nucleophile has formed a complete bond to. In this reaction sn 2+ change in sn 4+ it is called an oxidation reaction. Since there are 2 electrons needed, for one mole of sn, 1 faraday would only produce a half of a mole of sn.
Sn++ loses two electrons in a reaction. But it have complete it's octet so it does it by formi. The bond making and bond breaking actions occur simultaneously.
When sn 2+ changes to sn 4+ in a reaction (a) it loses two electrons (b) it gains two electrons (c) it loses two protons (d) it gains two The electronic configuration of an atom in the shell atomic model may be expressed by indicating the number of electrons in each shell beginning with the first. For example, with sn2+ the 2+ tells you that two electrons were lost.
When at atom gains electrons a negative ion is formed. The electron configuration of an element with an atomic number greater than 18 cannot be properly determined according to the bohr atomic model. The electronic configuration of sn is 5s25p4.
Sn 2+ loses two electrons in a reaction. The only way for that to occur is to reverse the tin reduction. See the answer see the answer see the answer done loading.
A +2 b zero c +4 d −2 medium solution verified by toppr correct option is c) sn 2+→sn 4++2e − sn 2+ loses two electrons in the reaction to form sn 4+ and its oxidation number changes from +2 to +4. Click here 👆 to get an answer to your question ️ how many valence electrons are in sn+2? What is the electron configuration for sn+2?when taking out the electrons, do you take from the s or psubshells first if they are at the same level?
This problem has been solved! There are 8 protons and 10 electrons in an oxide ion. Br2(l) +2e− → 2br−(aq), e∘ red = 1.08 v sn2+(aq) + 2e− → sn(s), e∘ red = −0.14 v the spontaneous reaction at 25∘c is that which has a standard cell potential e∘ cell = e∘ red +e∘ ox > 0.
But the more stability of sn(ii) compounds than sn(iv) compound can be explained by 1. How many protons and how many electrons are there in an oxide ion? As the nucleophile forms a bond with this carbon atom, the bond between the carbon atom and the leaving group breaks.
Electrons can be arranged correctly through orbits from elements 1 to 18. In the s n 2 reaction, the nucleophile approaches the carbon atom to which the leaving group is attached. What will be the oxidation number of tin after the reaction?
What is the electron configuration of tin, sn? Therefore, the order of the number of electrons in each shell of the tin (sn) atom is 2, 8, 18, 18, 4. Due to this stable electronic configuration, sn+4 is more stable than sn+2.
Tin is in the fifth energy level (row). Please answer asap thank you lexissixel lexissixel 12/14/2020 To write the configuration for the tin (sn).
When sn 2+ changes to sn 4+ in a reaction a it loses two electrons b it gains two electrons c it loses two protons d it gains two protons medium solution verified by toppr correct option is a) sn 2+→sn 4++2e −. 1s2, 2s2 2p6, 3s2 3p6 3d10, 4s2 4p6 4d10 ni^2+. Therefore, sn+2 lose 2 electrons to achieve full filled electronic configuration of sn+2 and become more stable.
The other major kind is s n 1. It can lose four electrons to achieve full filled configuration.

Electron Pushing In The Sn2 Reaction - Youtube
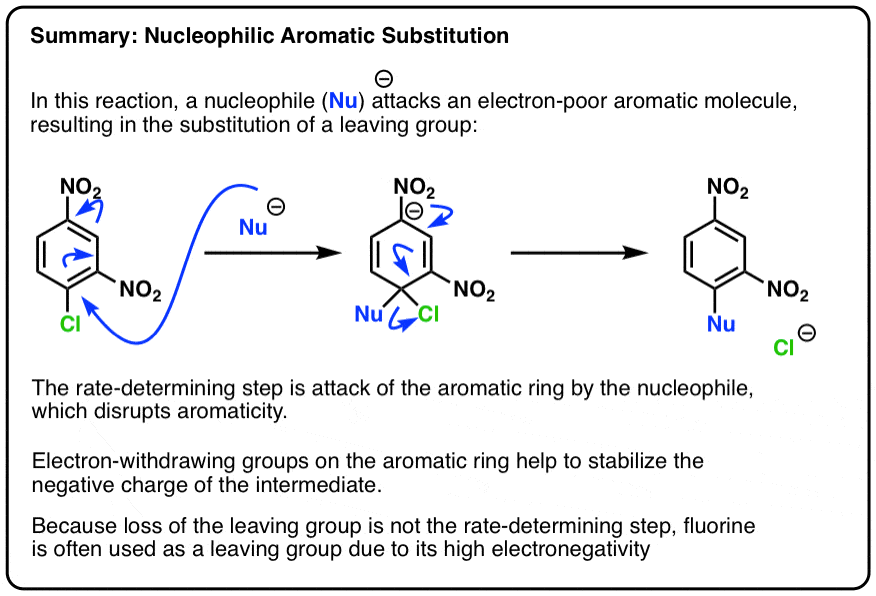
Nucleophilic Aromatic Substitution: Introduction And Mechanism