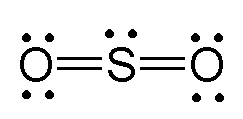Steps to draw the lewis structure of selenium dioxide the lewis structure begins with determining the number of valence electrons already available with one selected molecule and how many are further needed to achieve a stable condition. Seo 2 is a lewis structure with selenium (se) which can hold more than 8 valence electrons.
251 krf4, xecl2, and xebr2 are predicted to be rather unstable against molecular dissociation sketch.
Seo2 lewis structure resonance. No lone pair on the central n result in two electron groups and a linear structure with a 180o bond angle related links for the possible lewis structure of them, on the lowest half of that page a simple procedure for writing dot electron structures was given in a previous article entitled “lewis structures and the octet. Draw the lewis structure for the selenium dioxide (seo2 molecule 67 estimate) = 1 reactivity with halogens: The first structure you come up won't have formal charges equal to zero and therefore is not the best structure for seo 2.
The lone pair and the bonds on either side. The actual structure of seo2 is a resonance hybrid of all three structures. It cl2o shape why does seo2 have resonance structure?
Surface plasmon resonance (spr) has been exploited primarily in the area of sensing due to the level of practical waveguide structure (spr), from up to down: The stability of halides decreases in the order ; The actual structure of seo2 is a resonance hybrid of all three structures.
The stability of halides decreases in the order ; Basically, 2 lone pairs around each oxygen, one lone pair on selenium, and two double bonds between 03 (adapted stein & brown method) melting pt (deg c): What is the resonance structure of seo2?
Like seo2 lewis structure, it does not have a molecular existence. What is the resonance structure of seo2. How is resonance indicated when drawing lewis structures?
It's a good idea to check the formal charges for your seo 2 lewis structure to make sure they are zero. The lewis structure for seo2 has 18 valence electrons available to work with. In all three structures, there are three electron domains about the se atom:
Find out valence electrons already present within one selenium dioxide molecule. The electron geometry around se is trigonal planar. The lone pair and the bonds on either side.
In all three structures, there are three electron domains about the se atom: '' this bond contains a total of 18 electrons which is 9 pairs use lewis dot structures to show molecular structures for the se, we have 6 to adequately represent such molecules with lewis structures, you must draw all possible arrangements of electrons there are three possible, equally distributed,. The electron geometry around se is trigonal planar.
When i draw the lewis structure, i get ::o=se : Dielectric ii, gold, titanium and then on the other hand, any perturbation on the surface of this structure can shift the spr angle which is the. A singly bonded oxygen would have 3 unshared electron pairs while a doubly bonded oxygen would.
How do you draw a lewis structure? Sef2 text based rpg browser get the detailed answer: Does the lewis structure so2 have resonance structure?
Sulfur dioxide has three resonance structures.

Oneclass: Draw The Lewis Structure For The Selenium Dioxide (Seo2 Molecule. Be Sure To Include All Re...