Sometimes develop your own equations in a step by step manner. Its layered structure resembles that of the brucite polymorph of nickel (ii) hydroxide, but with half as many hydrogens.
The results showed that, with increasing loading of naoh, the h2s breakthrough.

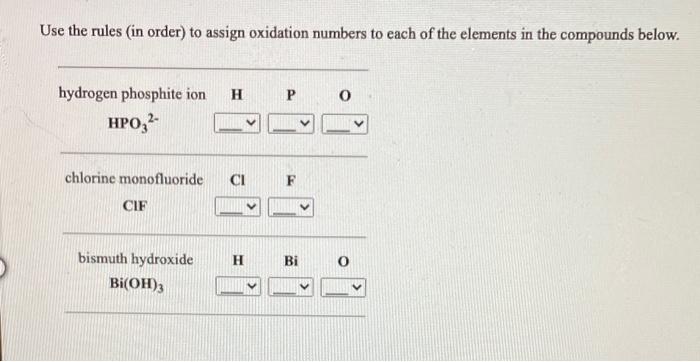
Oxidation number of hydrogen in hydroxide. How many hydroxide ions are needed to form water? The oxidation state defines the electropositive or electronegative nature of an element in a compound. And thus the oxidation numbers for hydrogen and oxygen in water are +i and −i i.
The ion has one oxygen atom and one hydrogen atom. Calculating the oxidation state of hydrogen in h 2 o (water) the oxidation state of oxygen(o. Four activated carbons of various origins were impregnated with different concentrations of sodium hydroxide and used as hydrogen sulfide adsorbents in an accelerated test.
Experimental and literature data on hydrated hydroxide melts are analyzed. The materials were characterized using nitrogen sorption, thermal analysis, and standard astm methods. Just follow the steps one by one.
The oxidation number of hydrogen is +1 except in q. Do this for water h −o −h, and we get h + +h o−; What is the oxidation number of lithium in lithium.
[i m directly doing above]. The oxidation number of hydrogen is + 1 except when it is bonded to metals in binary compounds (that is compounds containing two elements). The oxidation state or number is the charge on the individual ionic element.
The sum of the oxidation numbers of all the atoms in a neutral compound is 0. The oxidation number of h is +1 (rule 1). The hydrogen oxidation reaction (hor), which is a fast 1e− process, takes place at the anode, and the oxygen reduction reaction (orr), which is a slow 4e − process, takes place at the cathode.
Hydrogen is less electronegative than oxygen, and so will possess its usual +1state. Shreyash lavate studied computer science & chemistry at willingdon college, sangli 4 y related The oxidation number of hydrogen is +1 when it is combined with a nonmetal as in ch 4, nh 3, h 2 o, and hcl.
I reiterate that this is an entirely conceptual exercise. 2h + cuo → cu + h2o. The oxidation number of simple ions is equal to the charge on the ion.
The oxidation state of nickel is 3+. The rigmarole can sometimes help to balance redox equations. In a hydroxide (oh−)ion, we see that the total charge of the ion is −1.
[2] it can be prepared by the reaction of nickel (ii) hydroxide with aqueous potassium hydroxide and bromine as the oxidant: The charge on each of the elements defines how many electrons are lost or gained by the element by the ion. [3] 2 ni (oh) 2 + 2 koh + br 2 → 2 kbr + 2 h 2 o + 2 niooh
Oxidation number denotes the oxidation state of an element in a compound ascertained according to a set of rules formulated on the basis that electron pair in a covalent bond belongs entirely to a more electronegative element. That means, the total sum of the oxidation numbers of the elements present in the ion totals out to be −1. Do this again for hydroxide, we get h + +o2−.
[1] h 3 o + + oh − ⇌ 2h 2 o the equilibrium constant for this reaction, defined as kw = [h + ] [oh −] [note 1] The oxidation number of hydrogen. The oxidation number of hydrogen is + 1 except in binary compounds.
Solved Use The Rules (In Order) To Assign Oxidation Numbers | Chegg.com

Radicals A Radical Is A Group Of Combined Atoms That Behaves Like A Single Entity During A Chemical Reaction. Are Also Known As Polyatomic Ions. They. - Ppt Download