Molecular nitrogen, n2, is a neutral molecule, therefore it has an oxidation state of zero. Oxidation state of permanganate ion =oxidation state of manganese.

Oxidation Numbers For Hno3? Trust The Answer - Barkmanoil.com
The oxidation number of nitrogen in elemental nitrogen is zero.

Oxidation number of hn02. Hydrogen almost always has an oxidation number of +1. Only in compounds with other element the oxidation number changes. When it is bonded to fluorine (f) it has an oxidation number of +2.
Oxidation number of nitrogen in no 2 (nitrogen dioxide). Here it is bonded to. The hydrogen atom (h) exhibits an oxidation state of +1.
To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'calculate' (for example: Solve any question of hydrogen. Since the atom as whole is single negatively charged, following equation holds true:
The sum of the oxidation numbers of. Oxidation number of nitrogen atom. Let the oxidation number of n in n o 3 − be x.
When it comes to redox reactions, there are a few reasonably effective numbers, such as oxidation numbers. Oxidation numbers are used to track how many electrons are lost or gained in a chemical reactions. Nitrogen dioxide (no 2) is a molecule which contain two oxygen atom and one nitrogen atom.
Free atoms (h2) usually have an. Oxidation number of water = 0 water is neuter then oxygen of oxidation no. In a full sentence, you can also say h2o (water) reacts with no2 (nitrogen dioxide) and produce hno3 (nitric acid) and hno2 (nitrous acid) phenomenon after h2o (water) reacts.
Since the molecule consists of two nitrogen atoms, each of which must have the same. In general, most metals will have an oxidation number equal to their charge when in ion form. Assigning these numbers involves several rules:
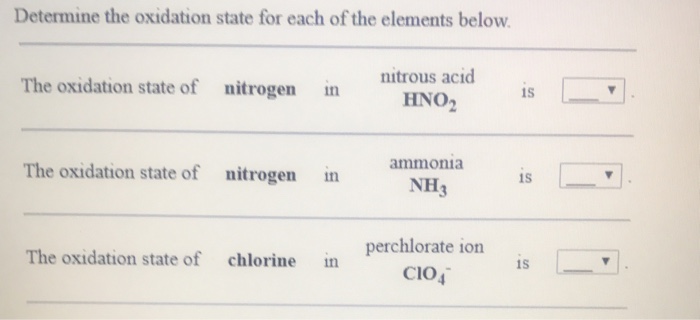
Solved Determine The Oxidation State For Each Of The | Chegg.com

How To Find The Oxidation Number For N In Hno2 (Nitrous Acid) - Youtube