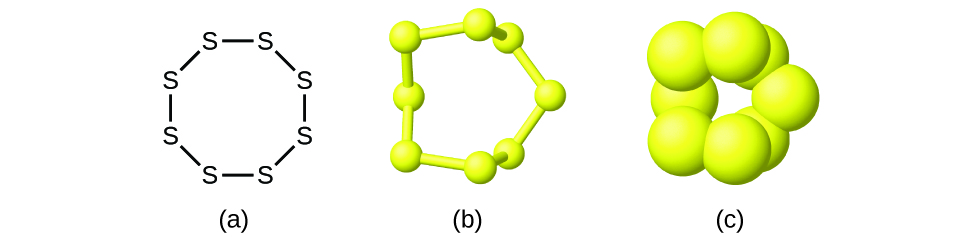
Sulfur (s) is an element that can never be overlooked. Which statement is true for one molecule of sulfur trioxide?
What Is The Molecular Mass Of Sulphur? - Quora
The name oxygen comes from the greek stems oxys , acid, and gennan, to form or generate. thus, oxygen literally means acid former.

One molecule sulfur. So yeah, we now did an option. Oxygen is the most abundant element on this planet. Liquid c7h8o is burned with oxygen gas to produce gaseous carbon dioxide and water vapor balanced…
So which one has more balls? Or we have one more love self try oxide which is which will be equal to 80 g. Basically, access which of the following substances have more wars of sulfur.
Multiply the number of moles by 6.022×10^23 atoms/mole. Yeah, yeah, to move verses. Our heaviest item how heavy is compound.
Number of atoms in one molecule of sulphur: 2 see answers advertisement advertisement missaann missaann But molecular weight is not an integer (except for carbon 12).
Reset 1 see answer kaitlyncolbird is waiting for your help. So in this case, there's going to be this one be here. There… get the answers you need, now!
Total number of atom in one molecule of sulfur is____. What is one atom of sulfur reacts with one molecule of oxygen to form one molecule of sulfur dioxide? So the number atoms in each sulfur is 8.
And option we have one more oxygen. It means one more of oxygen is equal to 32 g. Sulfur dioxide, so2, reacts with oxygen, o2, to form sulfur trioxide, so3 sulfur trioxide then… a:
In colour, it is bright yellow, and it has an extremely bad odour (like rotten eggs). Sulfur (s), also spelled sulphur, nonmetallic chemical element belonging to the oxygen group (group 16 [via] of the periodic table), one of the most reactive of the elements. Mahuyamukherjee555 mahuyamukherjee555 3 weeks ago science secondary school +5 pts.
Pure sulfur is a tasteless, odourless, brittle solid that is pale yellow in colour, a poor conductor of electricity, and insoluble in water. The molecular weight of oxygen (o2) is : One mole of so2 contains 1 mole of s atoms and 2 moles of o atoms, for a total of 3 moles of atoms.
There is one atom of sulfur and one atom of oxygen. 3 moles × (6.022×10^23 atoms/mole) = 1.807×10^24 atoms to four significant figures there are ~1.807×10^24 atoms in one mole of so2. Which statement is true for one molecule of sulfur trioxide?
Some basic concepts of chemistry book:aakash institute. Sulfur dioxide = so2 sulfur trioxide = so3 sulfuric acid = h2so4 silver sulfide = ag2s q: See answer (1) best answer.
I mean molecular mass of the given molecule is 80 g. Answered one molecule of sulphuric acid is made of 2 We have one mole of oxygen.
There is one atom of sulfur and three atoms of oxygen. There is one atom of sulfur and one atom of oxygen. Calculate mass of one molecule of sulphur dioxide (so_(2)) in gram.
In this case, which one's more? 1 mole of anything is 6.022×10^23 things, including atoms. One sulfuric (h2so4) molecule has 2 hydrogen atoms, 1 sulfur atom, and 4 oxygen atoms.
Four for most of us. Number of atoms in one molecule of sulphur a. You can also say one mole of sulfuric acid has two mols of hydrogen atoms, 1 mol of sulfur atoms, and 4 moles of oxygen atoms.
The earth's crust is 46.6% oxygen by weight, the oceans are 86% oxygen by weight, and the atmosphere is 21% oxygen by volume. We will update the answer very soon. Click here👆to get an answer to your question ️ number of atoms in one molecule of sulphur:
Our expert is working on this class x maths answer. It is located in group six or sixteen and period three of the periodic table. Hence option a is correct.
It's basically just going to be the same in both above, so no. Add your answer and earn points. There is one atom of sulfur and three atoms of oxygen.
In the periodic table, sulfur is found in group 16.