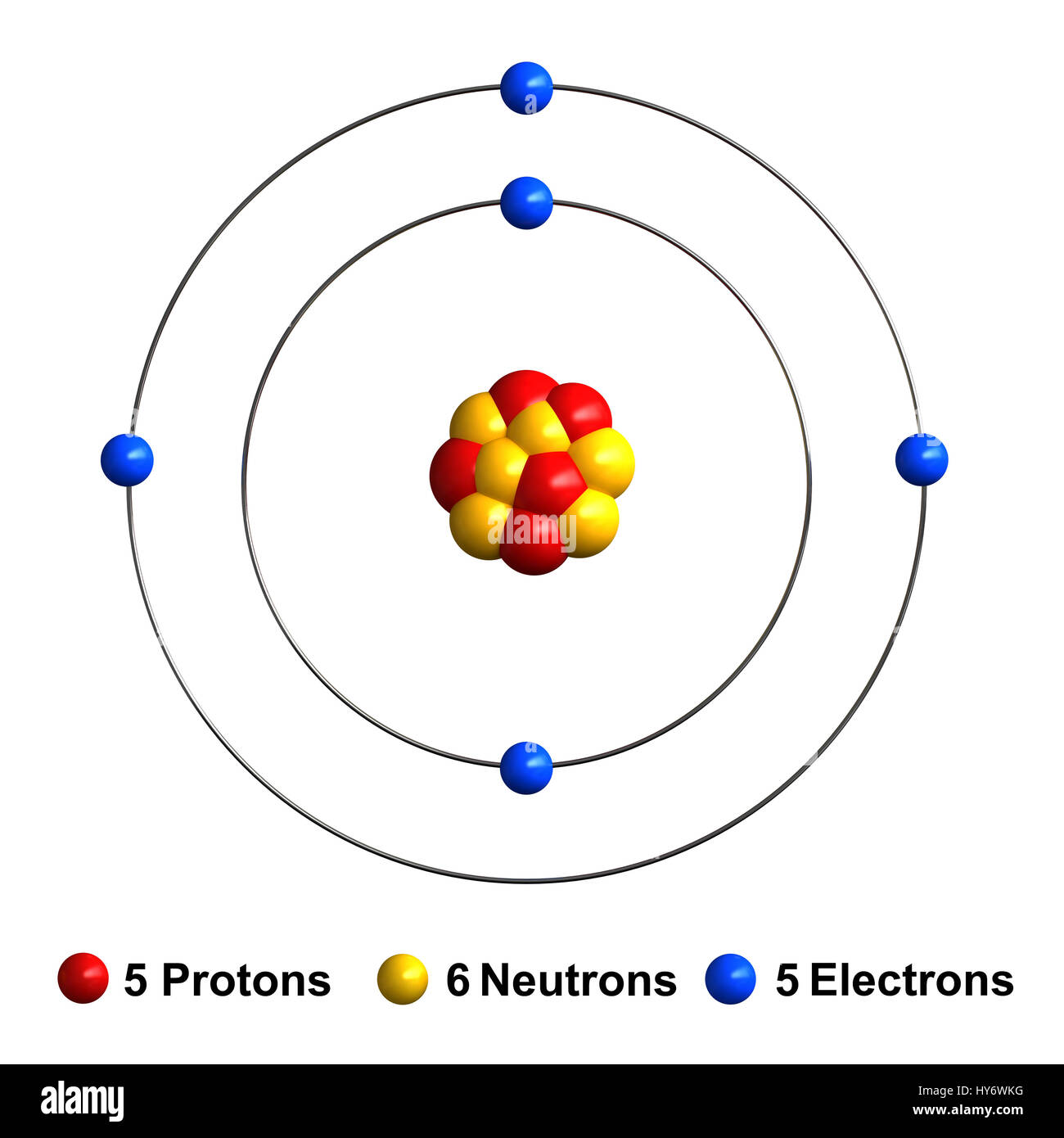
First, find the number of electrons an atom of boron usually has. Having 5 protons, it also has 5 electrons.
Boron Atomic Structure Hi-Res Stock Photography And Images - Alamy
When it is amorphous boron, it is discovered as a brown powder.
Number of electrons in boron. 1 see answer advertisement advertisement nagendra5760 is waiting for your help. Four electrons fill both orbitals 1 and 2. The electronic boron configuration is:
Concept of resistance & ohm’s law; You can see that it has 3 more positive charges than it usually has. 1s22s22p1 table 5.2 shows the electron
1 s 2 2 s 2 2 p 1 here we can see s First of all we have to look into electronic configuration of boron. Determine the valence shell and calculate the total electrons
We know that boron atoms have a total of five electrons. [1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f]. This means it has 5 protons.
Now, look at the charge. Boron is a chemical element with atomic number 5 which means there are 5 protons and 5 electrons in the atomic structure. That is, the first shell of boron has two and the second shell has three electrons.
How many electrons in boron outer shell. Since the number of electrons is responsible for the chemical behavior of atoms, the atomic number identifies the various chemical elements. The electron configuration of boron shows that there are two electrons in the k shell and three in the l shell.
Now, for the electron configuration of boron, the first 2 electrons will go in 1s orbital since s subshell can hold a maximum of 2 electrons. They are arranged with two electrons in the first energy shell and three in the second. If you look in the periodic table, you will notice that boron has 5 protons.
Boron has three electrons in its last orbit. The chemical symbol for boron is b. Boron is element number 5 in the periodic table.
In this case, the boron atom carries a positive charge. The valency of the boron is 3. So, it must have 5 electrons because it is a stable element and has no net charge.
The elemental boron chemical symbol is b. The number 11 represents the mass number which is the sum of protons and neutrons. The boron atom donates three electrons of the last shell to turn into a boron ion (b 3+ ).
The boron atom has only six electrons in its outer shell, leading to an electron deficiency. The electrons will be placed in different orbitals according to the energy level: The boron atom has a total of five electrons so, we have to put 5 electrons in orbitals.
3 each from the b atoms, and 1 each from the six h atoms. So, on a periodic table, boron has an atomic number of 5. As per the aufbau rule, the electrons will be filled into 1s orbital first then 2s, then 2p…so on.
Now, boron electron configuration b (5) = 1s22s22p1 (complete configuration). Boron is having atomic number 5 which means it is having five electrons in its orbits. So, usually, boron (b) has 5 electrons.
So two in the first and 3 in the second. 1s22s1 boron (atomic number 5) has five electrons. What is the number of electrons for boron?
That means it contains five protons and five electrons. The boron atom has only six electrons in its outer shell, leading to an electron deficiency. It is one of the lightest elements, with only five electrons, a nucleus of six neutrons, and five protons.
A chemist would say that the electron configuration of boron is 1s22s22p answer link 3 each from the b atoms, and 1 each from the six h atoms. And here boron is having three electrons in its outermost shell.
A boron atom is a neutral atom that has 5 atomic numbers which implies it has a total of 5 electrons. So let us write the electronic configuration of the boron atom. In order to calculate the number of neutrons:

Boron Bohr Model: Diagram, Steps To Draw - Techiescientist

B Boron Element Information: Facts, Properties, Trends, Uses And Comparison - Periodic Table Of The Elements | Schoolmykids