It resembles the configuration of the nearest inert gas i.e neon. To write the electron config all we have to do is add one electron from the configuration for i.
Properties and uses of nitrogen it is the lightest.

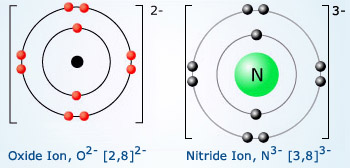
Nitride ion electron configuration. Here, the total number of electrons in the ion is 10. The atomic number of lithium is 3. This negative nitride ion(n 3− ) has seven protons, seven neutrons, and ten electrons.
Nitrogen forms a nitride ion with the electron configuration 1s2 2s2 2p6 write the formula of the nitride ion. Here, the electron configuration of nitride ion(n 3−) is 1s 2 2s 2 2p 6. 1 nitrogen has an atomic number 7.
The four quantum numbers are principal quantum number which denotes the shell, azimuthal. Therefore, a lithium atom will have two electrons in the first shell and one in the 2nd shell. What is the overall charge on this ion?
The nitride ion is n − 3 the original electron configuration for nitrogen is 1 s 2 2 s 2 2 p 3 in order to fulfill the octet rule, the nitrogen atom would take on three additional electrons giving. If you are still not getting the nitrogen electron configuration of the element nitrogen then, the full electronic configuration of nitrogen is written as the following; That is, the number of electrons in lithium is 3.
We’ll also look at why nitrite forms. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 and that is the electron. The nitride ion, n3ˉ, has an electron arrangement of 1s2, 2s2, 2p6 the elemental nitrogen, n, has an electron arrangement of 1s2, 2s2, 2p3.
The formula for nitride ion is n 3 −. A nitride ion has 7 protons, 8 neutrons, and 1 0 electrons. What is the electron configuration for a nitride ion?
A nitride ion is formed when a nitrogen atom having 7 electrons acquires 3 more electrons to get the configuration [he]3s2 2p6.