A −1.86 oc b −3.72 oc c +1.86 oc d +3.72 oc medium solution verified by toppr correct. This aspect is important when dealing with colligative properties because the number.

Freezing And Thawing Points Of Samples Saturated With Different Nacl... | Download Scientific Diagram
This content is pdf only.
Nacl freezing point. The freezing point depression is calculated by the following formula: Only the dissolved salt contributes to depression of freezing point. Freezing point depression is a colligative property, meaning that the lowering of the freezing point does not depend on the kind of particle, only on the number of.
An aqueous solution of sodium chloride freezes below 273 k. There‘s this thing called ebullioscopy. Maximum solubility of nacl in water is 35.5 grams in 100.
These results suggest that the nacl dissolution may occur well below the freezing point at the ice/ nacl interface, and may be mainly driven by the interaction. Reason the lowering of vapour pressure of a solution causes depression in the freezing point. In this case, the only information you have is that a given sodium chloride solution has a freezing point of −5.58∘c.
Nacl solutions is shown in figure 2. Δ tf = m x. When a solution nacl (aq) is freezing there are two ions interfering / preventing the solution to freeze, while when cacl2 (aq) is freezing, three ions are contributing to the depression of.
As you know, the freezing point of a solution is a colligative. The change in freezing point = es * n, where es is a constant (for water 0.515) and n is the mola l ity which means mol/kg, not per litre. Suggest corrections 1 similar questions q.
The freezing point of 1 molal nacl solution assuming nacl to be 100% dissociated in water is: The equation for calculating depression of freezing point is: Commonly used sodium chloride can depress the freezing point of water to about −21 °c (−6 °f).
This plot shows our measurements for the freezing point of solutions up to a concentration of 20 wt% nacl up to 69 mpa using the methods described. For example, calculate the freezing point of the solution prepared by dissolving 4.80 g nacl in 25.0 g of water. Adding 31.65 g of nacl to 220.0 ml of water will lower the freezing point by 9.21 °c.
The correct option is c 5m nacl solution since δt f for 5m nacl will be higher than for 2m, 5m nacl solution freezes at a lower temperature. Calculate the decrease in the vapor pressure of water at 25°c caused by this concentration of nacl, remembering that 1 mol of nacl produces 2 mol of solute particles. Assertion when nacl is added to water, a depression in freezing point is observed.
Explain the lowering in freezing points of water with the help of a suitable diagram. Limitations of freezing point depression calculations calculating freezing point depression. Economic geology (1988) 83 (1):
This happens because salt completely dissociates in aqueous solution, while sugar does not. A nacl solution was placed into room temperature water and heated until all the sodium chloride had dissolved to. If the road surface temperature is lower, nacl becomes ineffective and other salts are used,.
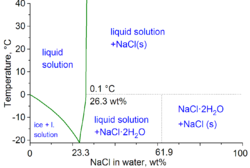
This value is the average between the values of the lowest. Example 13.8.3 in example 13.8.1, we calculated that the vapor pressure of a 30.2% aqueous solution of. The freezing temperature at this concentration for the ice/nacl (aq) system is tf (solution) = 248.25 ± 0.25 k.
Hence a 1.00 m nacl solution will have a boiling point of about 101.02°c.