Nov 2, 2015 if we build the mo diagram for n2, it looks like this: Molecular orbitals homonuclear oxygen atom diagram.
Molecular Nitrogen And Related Diatomic Molecules
Home / structure and bonding / atomic orbitals / molecular orbitals in carbon monoxide.
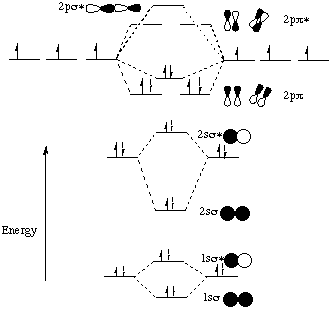
N+ molecular orbital diagram. [189] have constructed molecular orbital and state correlation diagrams for the ortho photocycloaddition of benzene to ethene. The net contribution of the electrons to the bond strength of. Boxes, or horizontal lines represent the orbitals, arrows represent the electrons, and if an orbital is full, the electrons must be of opposite.
To write the orbital diagram of nitrogen (n), you have to do the electron configuration of nitrogen. The main features of molecular orbital theory for metal complexes are as follows: They weren't drawn that way.
This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the. 1.the atomic orbital of the metal center and of surrounding ligands combine to form new orbitals, known as. Of the donor ( n representing the hydrogen atom 1 s orbital), of the acceptor (bonding χ and antibonding χ *.
Draw be sure your diagram contains all of the electrons in the. 100% (1 rating) transcribed image text: The orbital for which the value of (n + l) is lower is the low.
This image shows the molecular orbitals of nitric oxide and the types of bonds present. Nitrogen orbital diagram 1s is the closest and. Click here👆to get an answer to your question ️ 17 #868132 in the molecular orbital diagram for the molecular ion, n,, the number of electrons in the o2p molecular orbital is?
A molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear. The molecular orbital correlation diagram. Draw the molecular orbital diagram of n 2, n 2+ n 2−.
Molecular orbital diagrams this scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the. An orbital box diagram can be written as well. Which has been discussed in detail above.
You can see that co is not (as it has zero unpaired electrons), but no is (it. (b) this plot of the square of the. 2.41.1 the molecular orbital the molecular orbital (mo) is the basic concept in contemporary quantum chemistry.
A molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear. Write their electronic configuration, find the bond order and predict their magnetic behaviour. A schematic representation of the molecular orbitals and their energies:
Van der hart et al. The molecular orbital (mo) electron diagram for the n2 molecule. (a) this diagram shows the formation of a bonding σ1smolecular orbital for h2as the sum of the wave functions (ψ) of two h 1s atomic orbitals.
The energy of an orbital is calculated from the value of the principal quantum number ‘n’ and the azimuthal quantum number ‘l’. Arrange the above in increasing order of. First though, notice that the p orbitals are supposed to be degenerate.
The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals.

Using The Mo Diagram Of "No", Calculate The Bond Order. Compare It To "No"^(+)? | Socratic
