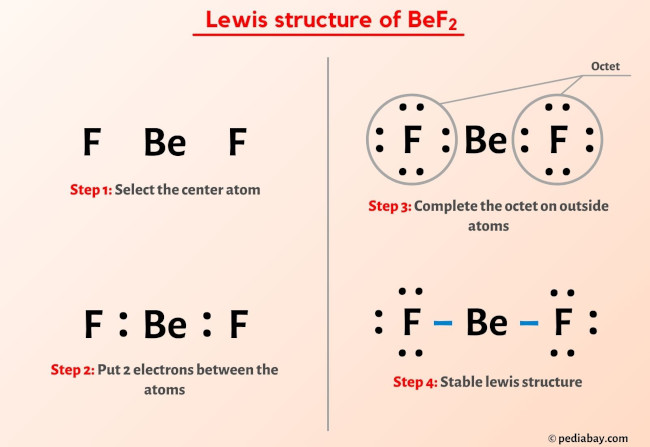
Xe is a noble gas and f is a halogen. Each fluorine atom has seven valence electrons, but as there are two fluorine atoms, we will multiply the number by 2.

How Many Dots Would Be Placed Around Fluorine In A Lewis Electron Dot Diagram? (A) 2 (B) 7 (C) 9 (D) 19 | Homework.study.com
In the lewis structure of f 2 o, two fluorine atoms are joint with center.
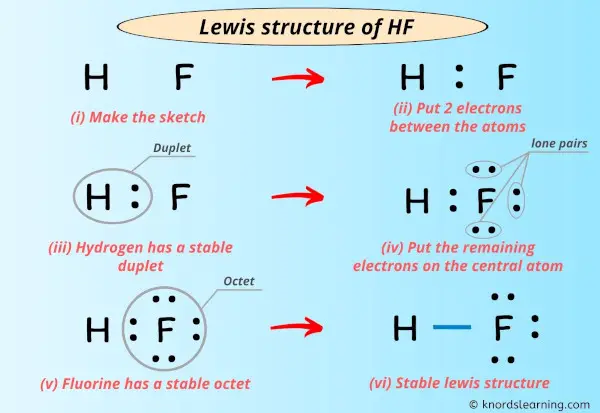
Lewis structure for f-. We can hence confirm that we have got. Ch3f lewis structure ch 3 f (fluoromethane) has one carbon atom, three hydrogen atoms, and one fluorine atom. Everything you need to teach lewis structures as an introduction or review is right here including the lesson, a student lesson handout, an interactive game of lewis war!
So in total we have 42 + 8 = 50. To use the lewis structure calculator follow these steps: 1.1 write lewis structures for each of the following:
As we can see, the elements are present in their least possible formal charge values. So xe has 8 valence electrons and f has 7. Steps to follow for drawing the of2/f2o lewis structure 1.
Considering there are 6 fs, that's 6 x 7 = 42 valence electrons from fs. The formal charge is an indicator of this stability. In short, these are the steps you need to follow for drawing a lewis structure:
Lewis structure stability is based on the internal charges of an atom or molecule. Let us find out the. Fcl does not exist as a molecule.
Lewis structure of oxygen difluoride (f 2 o) oxygen difluoride (f 2 o) has two fluorine atoms and one oxygen atom. So we have 14 valence electrons from fluorine. * hydrogen atoms are always terminal (only one bond).
Enter the formula of the molecule in the field provided for it. There is only one single bond between two fluorine atoms and three lone pairs on each fluorine atoms. For example, if we want to obtain the lewis structure of the sulfate ion,.
In the lewis structure of ch 3 f, there are four single bonds. The lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between the atoms). What is the lewis structure of fcl?
The reactivity of the compound is also consistent with an electron deficient boron. Caf 2 lewis structure formal charge. The lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between the atoms).
Count total valence electron present in of2 in the first step, you need to find how many valence electrons are present in the of2. Ch2f2 lewis structure, molecular geometry, hybridization, and polarity ch2f2 or difluoromethane or difluoromethylene is an organic compound of the haloalkane family. Write the correct skeletal structure for the molecule.
It is very easy to draw the f 2 molecule lewis structure.
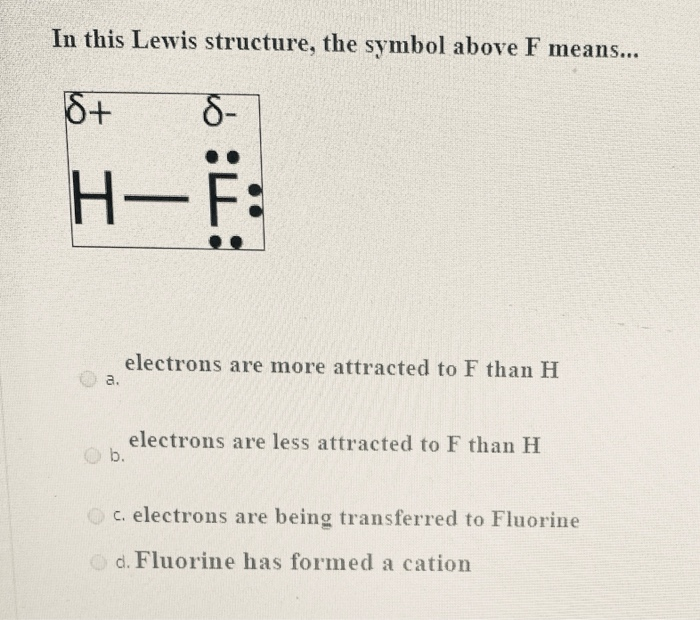
Solved In This Lewis Structure, The Symbol Above F Means... | Chegg.com