So carbon is sp hybridized with zero lone pairs, thus, it is linear shape. The molecular shape of hcn is linear.
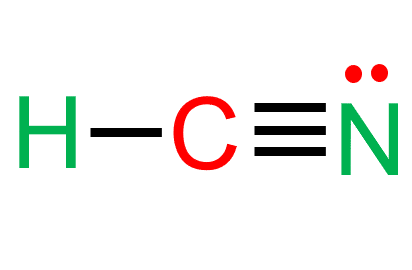
Hcn Lewis Structure & Molecular Geometry - What's Insight
Carbon has an electronegativity of 2.5, hydrogen’s electronegativity is 2.1, and nitrogen has an electronegativity of 3.
Is hcn linear. Thus conferring an overall partial positive charge on one end of the molecule and a partial negative on the other end. Nice first post ever, way to pitch in. Because hydrogen and nitrogen are usually far apart, hcn takes on a linear shape.
Any covalent bonds is basically consisted of two electrons from each of the bond forming atoms. The molecular geometry and electron geometry will both be linear molecular geometry. Hcl ( hydrogen chloride ) is a binary compound consisting of two atoms, a hydrogen ( h ) and a chlorine (cl ) atom.
Hcn, or hydrogen cyanide, is a colorless, highly toxic, and combustible chemical compound. Hence the molecule has two electron pairs and is linear. Hcn, with three atoms could be planar as three points are all that is needed to define a plane.
Hydrogen cyanide has linear molecular geometry with bond angles of 180 degrees. Hcn polarity hcn in a polar molecule, unlike the linear co2. It has a polarity bond of 2.98 d, ten valence electrons.
Join / login >> class 10 >> general knowledge >> basic science >> basic chemistry >> is hcn linear, tetrahedral, or trigonal. The polarity of hcn is 2.98 d. In c2h6, if you draw it out, you all the carbon atoms are single bonded to 4 different atoms, making it sp3 hybridized, thus tetrahedral shape.
Donald montecalvo suggested i reconsider my answer. Is h c n linear, tetrahedral, or trigonal planar? Because of its electronegative value, nitrogen tries to pull electrons to.
Hcn and compounds formed with this are useful for many chemical reactions. The angle generated by two covalent bonds is known as bond angles. Hcn is linear because carbon forms a single bond with h and a triple bond with n.
Hcn is a planar molecule. It has a polarity bond of 2.98 d, ten valence electrons. A pyramid is a generic term and can have any number (>3) of plane faces.
A pyramid whose base has n sides has n+1 faces. Hydrogen cyanide is a linear molecule. It is also used in the production of plastics.
It consists of two polar bonds whose polarities line up in the same direction. However, it is not planar, it is linear since all three atoms lie on a line. Click here👆to get an answer to your question ️ is hcn linear, tetrahedral, or trigonal planar?
Central atom is carbon and it is sp hybridized with a linear structure. Hcn sigma and pi bonds. At 25.6 °c, it boils slightly above room temperature.
<<strong>hcn</strong> bond angle is 180 0. A triangular pyramid has 4 faces, a rectangular one has 5, and so on. It is slightly polar as nitrogen tries to pull the electrons to itself due to its electronegative value.
What is the molecule polarity of hcn? As hydrogen and nitrogen tend to be far from each other, hcn forms a linear shape. Is hcn planar or nonplanar?
What is the molecular polarity of hcn? Does hcn have hydrogen bond? How many plane shapes in a pyramid?
From the point of hybridization the bond connectivity of central atom with each of the atom is clear. The molecule hydrogen cyanide, hcn, does not have hydrogen bonding. Uses of hcn hcn is used in the preparation of acrylonitrile which is further used in the manufacturing of synthetic rubbers, acrylic fibers.
Hcn shape as both hydrogen and nitrogen are placed far from each other at bond angles of 180 degrees, it forms a linear shape. And, formally, he is correct. Hcn, or hydrogen cyanide, is a polar molecule because there is a large electronegative difference between the n and h across the linear molecule.
How To Draw Hcn? (With Pictures, Videos) Answermeup

Hcn Lewis Structure, Molecular Geometry, Shape, And Polarity