Get 1 free homework help answer. A) which is more soluble in water, benzene ( c6h6 c 6 h 6) or ethanol ( c2h5oh c 2 h 5 o h ).
Benzene - Uses, Production, Formula, Structure
Benzene because it can form.

Is benzene c6h6 soluble in water. Calculate the mass percentage of the resulting solution. Weight of solute in 2nd solution = 400 × = 160 gms. Total weight of solute = 75 + 160 = 235 gms.
Benzene is not soluble in water. Is benzene (c6h6) soluble in water? So benzene has some solubility in water.
Water is a polar molecule because one side of the. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. C6h6 soluble or insoluble in water.
Cyclohexane (c6h12) is soluble in benzene (c6h) and insoluble in water. Benzene because it can form london forces with water molecules. Benzene is insoluble in water according to the reasons mentioned below:
National center for biotechnology information. By that definition, benzene is insoluble in water. Does benzene soluble in water.
This all depends on the structure and the arrangement of the atoms in a molecule so as to the net effect because of the overall symmetry give rise to what we call polar and no polar materials (liquid in this case). The question then is, why is benzene relatively insoluble in water? This is nitrobenzene, and it is a covalent compound which is a reason that it is soluble in benzene, alcohol etc but insoluble in water.
Benzene is a natural constituent of crude oil and is one of the elementary petroc… At higher temperatures, solubility increases. The reason is pretty simple, water is a polar liquid as in there.
So, we can say c6h5no2 is soluble in benzene but almost insoluble in water. The solubility of benzene in water is 1.79 g/l (about 0.02 mol/l) at 15 °c. Given a solution is obtained by mixing 300 gms of 25% solution and 400 gms of 40% solution by mass.
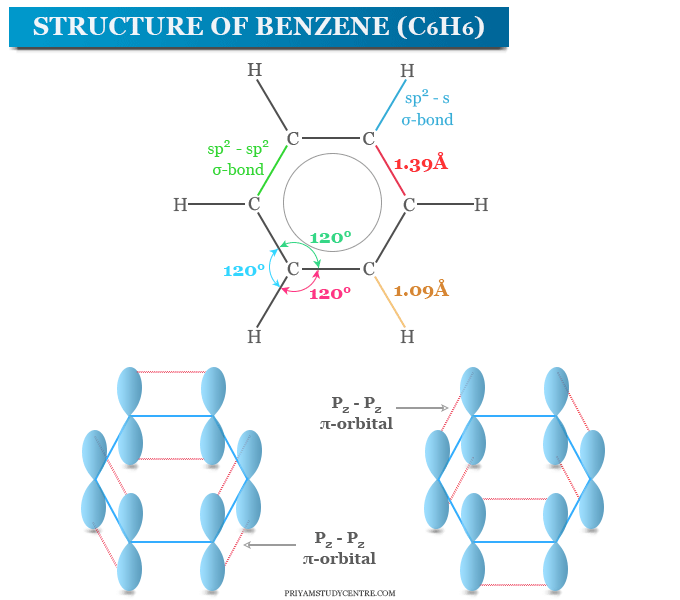
The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Solubility of naphthalene in benzene 20 related questions found is benzene polar or nonpolar? It’s used to make synthetic fibers, detergents and even drugs.
This is because benzene has a lower density than water lower heat capacity than water lower boiling point than water lower polarity than water. Weight of solute in 1st solution = 300 × = 75 gms. For unlimited access to homework help, a homework+ subscription is required.
Benzene is an organic chemical compound with the molecular formula c6h6.

What Is Benzene? - Uses, Structure & Formula - Video & Lesson Transcript | Study.com