What is the molecular shape of the following ions? Sf4 or sulfur tetrafluoride is a compound that has a distinct odor of sulfur or rotten eggs.
How Can I Predict The Bond Angles For If_4""^(-)? | Socratic
Understanding the molecular structure of a compound can help determine the polarity, reactivity, phase of matter, color, magnetism, as well as the biological activity.
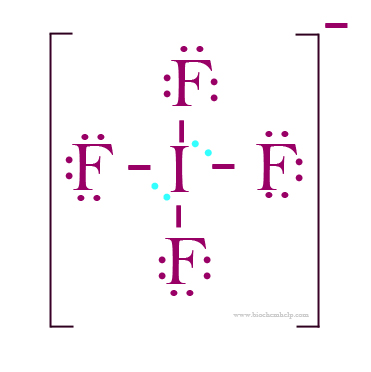
If4- molecular geometry degree. The i f 4− has a total number of 36 valence electrons: The lone pair on the central atom leads to the change in the bond angles from 120 degrees to 102 degrees for equatorial fluorine atoms and 173 degrees instead of 180 degrees for axial fluorine atoms. Under ordinary conditions, it appears like a colorless crystalline.
Calculate the total number of valence electrons. Looking at the positions of other atomic nuclei around. 60degree 90 degree 109.5 degree 120 degree deviation from ideal angle(s).
Draw the lewis structure.count the number of electron groups and identify them as bond pairs of electron groups or lone pairs of electrons. 1 the ground state electronic configuration of iodine = [kr]4d105s25p5 = [ k r] 4 d 10 5 s 2 5 p 5. This compound is generally identified as being a colorless gas.
The given cation has one iodine and four fluorine atoms, as well as a lack of a single electron. The iodine atom will be the central atom. It will form four single bonds with the fluorine atoms, for.
Recently i came across a question asking for the geometry of the aforementioned molecule. The molecular weight of this compound is calculated to be 108.6 g/mol. See also where are earthquakes and volcanoes most commonly found?
90 degrees is the value of the smallest bond angle in. Same as the other xenon fluorides, the xenon tetrafluoride has an exergonic formation. Thus, the total number of valence electrons.
90 degrees is the value of the smallest bond angle in. The electron geometry for the is also provided. Iodine has 7 valence electrons in its ground state.
The xef4 has a solid white appearance and has a density of 4.040 g cm−3 in a solid form. If4 (iodine tetrafluoride) has an octahedral electron geometry but the molecular geometry states that the atoms take a square planar shape. What is if4 molecular geometry?
7 from iodine, 7 from each of the four fluorine atoms, and 1 from the negative charge.

If4- Lewis Structure: How To Draw The Lewis Structure For If4- - Youtube

What Is The Shape Of If4+? A. Octahedral B. Seesaw C. Square Pyramidal D. Tetrahedral E. Trigonal Pyramidal | Homework.study.com