Hydroxide is also called hydroxyl or hydroxyl radical or hydroxide ion. 🛠️ calculate oxidation numbers instructions use uppercase for the first character in the element and lowercase for the second character.

Oxidation Number For Oh- . Oxidation State Of Hydroxide Ion. Oxidation State Of Oh- . Oh - Oxidation - Youtube
Nickel oxide hydroxide nickel oxide hydroxide is the inorganic compound with the chemical formula nio (oh).

Hydroxide oxidation number. When it is bonded to fluorine (f) it has an oxidation number of +1. It is a black solid that is insoluble in all solvents but attacked by base and acid. Assigning oxidation numbers to organic compounds
Peroxides include hydrogen peroxide, h 2 o 2. The hydrogen carries a negative electric charge. Examples c6al2o12 nibr2 sb2o3 c3h8 (c2h2) ph4 + ocs cr nicl2 (h2o)4*2h2o (nh2)2 ni (cn)2 crbr3 (cu (nh3)4)so4
Let #x# be the oxidation number of. It consists of hydrogen and an oxygen atom which are held together by a covalent bond. Oh − is a diatomic anion with the chemical name hydroxide.
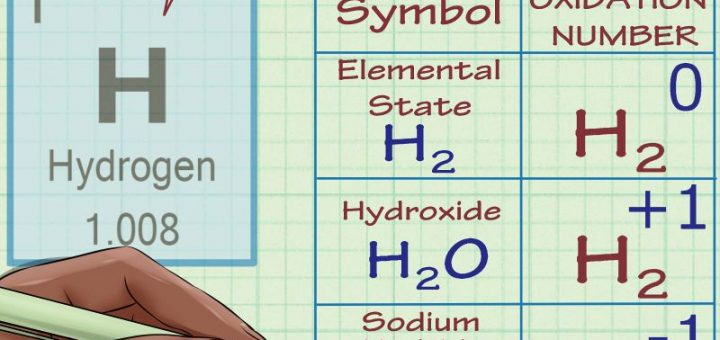
The oxidation number of h in naoh is +1. This is an electrically neutral compound, so the sum of the oxidation states of the hydrogen and oxygen must be zero. Oxidation states simplify the whole process of working out what is being oxidised and what is being reduced in redox.
Oxidation numbers of each element in cadmium hydroxide. The oxidation number of h is +1 when combined with more electronegative elements (e.g. The ion has one oxygen atom and one hydrogen atom.
The reaction between sodium hydroxide and hydrochloric acid is: The algebraic sum of the oxidation numbers of elements in a compound is zero. The oxidation number of na in naoh is +1.
Hydrogen is less electronegative than oxygen, and so will possess its usual #+1# state. Fe, au, co, br, c, o, n, f. Here it is bonded to so the oxidation number.
The algebraic sum of the oxidation states in an ion is equal to the charge on the ion. Checking all the oxidation states: Best dj software for beginners mac.
Explaining what oxidation states (oxidation numbers) are. Since two hydroxide (oh − −) ions are required to cancel the. See the answer see the answer done loading.
What is the oxidation number of lithium in lithium. Naoh + hcl nacl + h 2 o. Contents 1 related materials 2 synthesis and structure
Copper,in nature,mainly exhibits two oxidation states.it can either have a +1 state, or a +2 state, depending on several factors.

How To Find The Oxidation Number For Fe In Fe(Oh)3 - Youtube
Hydroxy Compounds, Rules For Calculating The Oxidation Numbers | Science Online