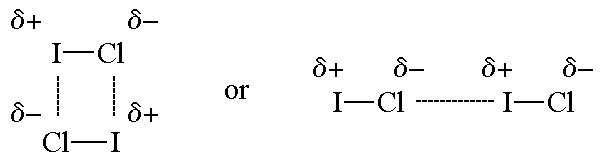This alignment also reduces the repulsions between the molecules. The molecules with dipole moments are known as polar molecules.
You need to figure out the shape of the molecule and see whether the charge is more concentrated on one end of the molecule due to electronegativity, eg.
How to tell if somethin ghas a dipole dipole force. What makes something dipole dipole? Dec 11, 2017 you have a dipole moment when there is a difference in electronegativity between two atoms. A big atom has many electrons moving around a large volume, so at any instant in time it is more likey to
Secondly lets clear the difference of ion ion forces. This means that the electrons hang around the cl more than the h. Unequal sharing of electrons results in opposite charges on the parent atom, forming permanent dipoles.
They are much weaker than ionic or covalent bonds and have a significant effect only when the molecules involved are close together (touching or almost touching). The oxygen atom in the water molecule has a slight negative charge and is attracted to the positive sodium ion. One end of the molecule is slightly positive and the other is slightly negative.
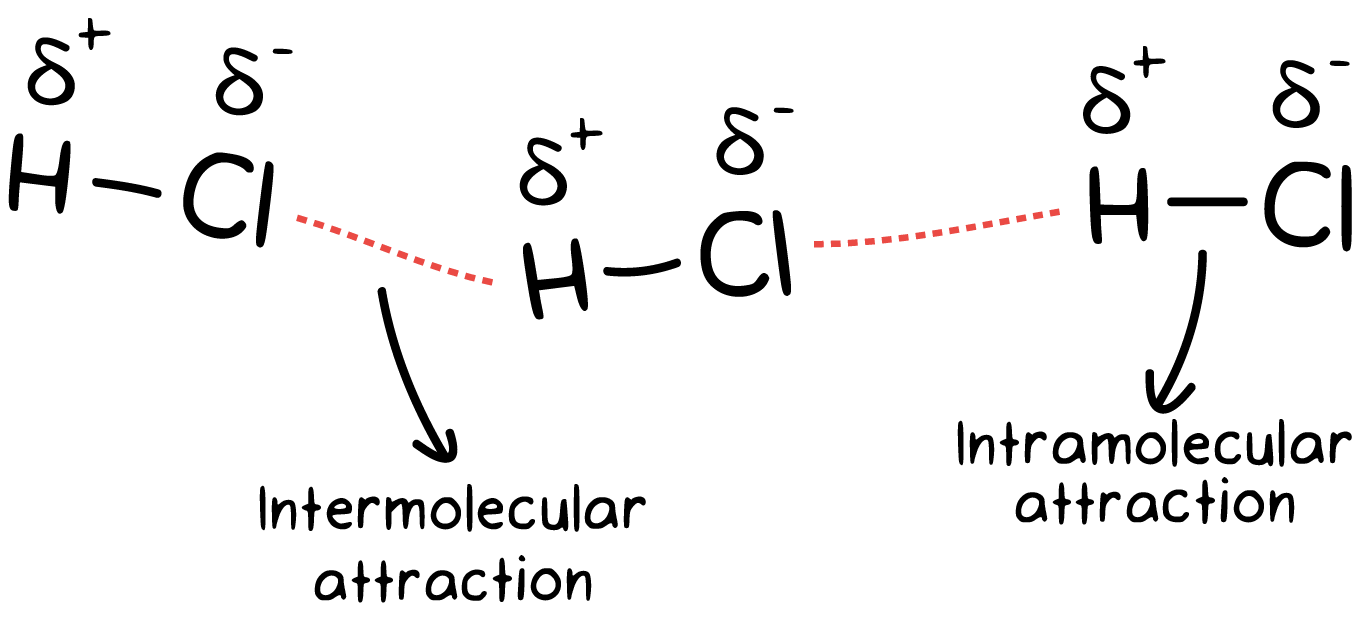
Above the ground has a potential energy equal to its mass x acceleration due to gravity x its height above the ground (i.e., \(mgh\)). The prefix di means 2, and so there has to be two poles. These forces are weak compared to the intramolecular forces, such as the covalent or ionic bonds between atoms in a molecule.
The direction and magnitude of polarity in a bond are given. This potential energy that an object has as a result of its position can be used to do work. Dipolar or polar molecules are the molecules that posses an electric dipole.the dipoles of some molecules depend on their environment and can change substantially when.
When forming this type of force, the polar molecules tend to be aligned so that the attraction between the molecules is maximized by reducing the potential energy. Another example the bigger the atoms or molecules involved, the larger the force of attraction is going to be. These forces exist between two ions in a formula unit as the name suggests so it is easy.
Note, these must be for solutions (and not pure substances) as they involve two different species (an ion and a polar molecule). We can depict this very simply as an oval with one positive side and one negative. For instance we could use a pulley system with a large weight held above the ground to hoist a smaller weight into the.
Therefore, h is left with a partially positive charge. The molecule is said to be a dipole.a dipole molecule is a molecule that has two (di) poles. First of all lets clear it that dipole dipole and london dispersion forces exist between two molecules and not between atoms inside a molecule.
Intermolecular forces are the forces of attraction or repulsion which act between neighboring particles (atoms, molecules, or ions). Dipoleany molecule that has both slight positive and negative charges on either end. N a + ↔ ( h 2 o) n figure 11.2.
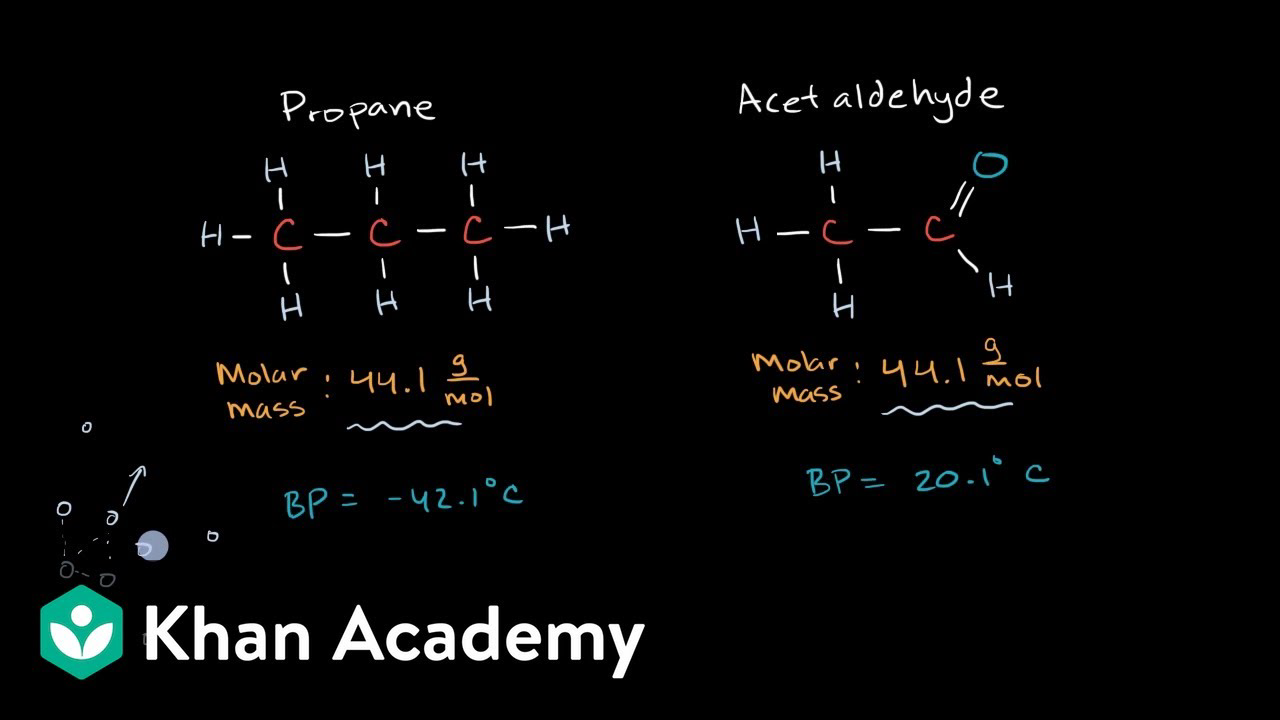
If the molecule is polar or has polar. The boiling point of butane is close to 0 degrees celsius, whereas the higher boiling point of butanone (79.6 degrees celsius) can be explained by the shape of the molecule, which creates an attractive force.

Dipole Dipole Forces Of Attraction - Intermolecular Forces - Youtube