Posted by 7 years ago. Then subtract 1 electron from 5.
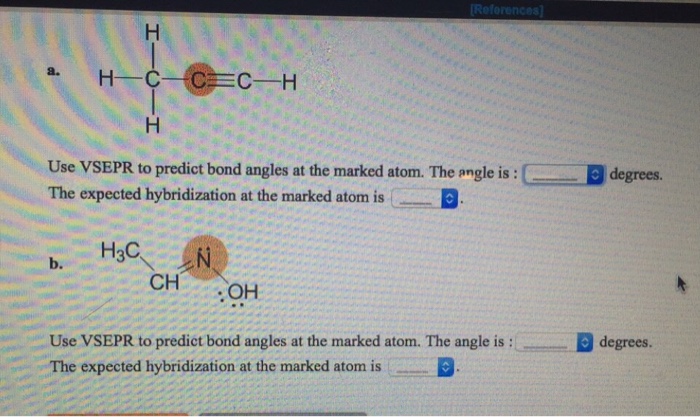
Solved Use Vsepr To Predict Bond Angles At The Marked Atom. | Chegg.com
How do you draw vsepr shapes?
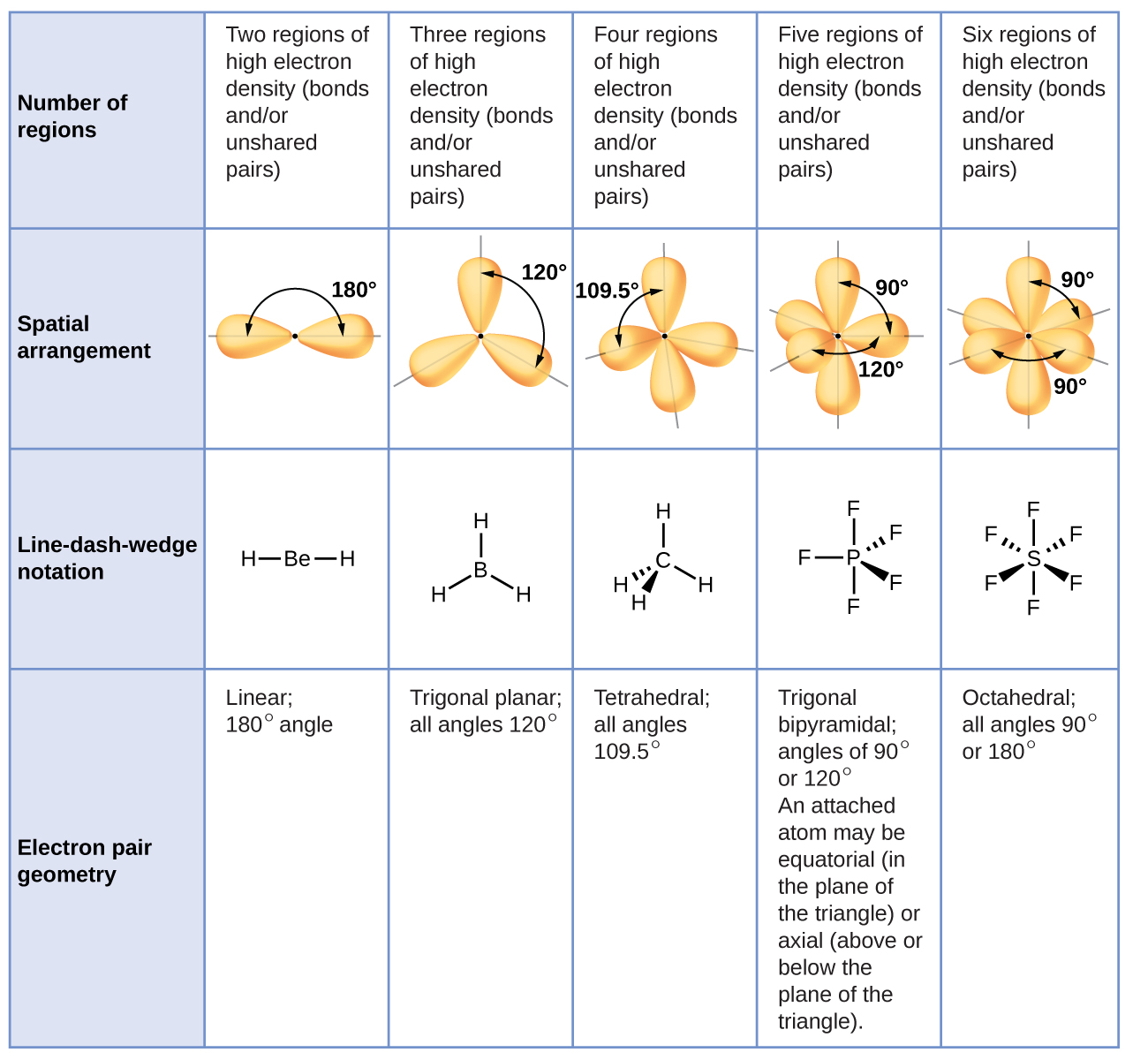
How to predict bond angles using vsepr. From the bp and lp interactions we can predict both the relative positions of the atoms and the angles between the bonds, called the bond angles. The following procedure uses vsepr theory to determine the electron pair geometries and the molecular geometries: Using vsepr theory, we predict that the two electron groups arrange themselves on opposite sides of the central atom with a bond angle of 180°.
It is a remarkably simple device that utilizes a simple set of electron. Im pretty confused by the vsepr theory when the molecule has double. 2 a multiple bond (double bond or triple bond) counts as one bond in the vsepr model.
Let's take a look at this question.we need to compare the bond angles of 4 pairs of substances and determine which pair has an increase in bond angle.that's. How to determine bond angles for c2br4 using vsepr. Count the total number of electrons the other atoms have and utilise them to form bonds with the centre atom.
Write the lewis structure of the molecule or polyatomic ion. 3 a = central atom, x = surrounding atoms, e = lone pairs 4 molecules with this shape are nonpolar. The vsepr theory or the valence shell electron pair repulsion theory is used in chemistry to predict the geometry of molecules from the number of electron pairs surrounding.
Add or subtract electrons for charge (see top tip) divide the. The postulates of vsepr theory are as follows: Ccl4 has no lone pairs on the central carbon and is therefore of tetrahedral geometry.
To get the valence shell electron pair number, or vsep. Use this number to predict the. For main group compounds, the vsepr method is such a predictive tool and unsurpassed as a handy predictive method.
Count the valence electrons of the central atom. Determine the number of lone pairs on the central atom from the lewis structure. How can i predict the bond angle?
Divide the total of these by 2 to find the total the overall electron total; The shape of a molecule is determined by the number of valence shell electron pairs. If number of electron pairs the central atom is negative, 6.
Add one electron for each bonding atom. Add an electron for each bonding atom. The main idea of vsepr theory is the repulsion between pairs of electrons (in bonds and lone pairs).
To use a vsepr table, first determine the coordination number or number of electron pairs. Vsepr theory can be used to predict the shapes and bond angles of various molecules with high accuracy. These pairs can be bonded or.

Vsepr Theory + Bond Angles - Mcat Lec - Youtube
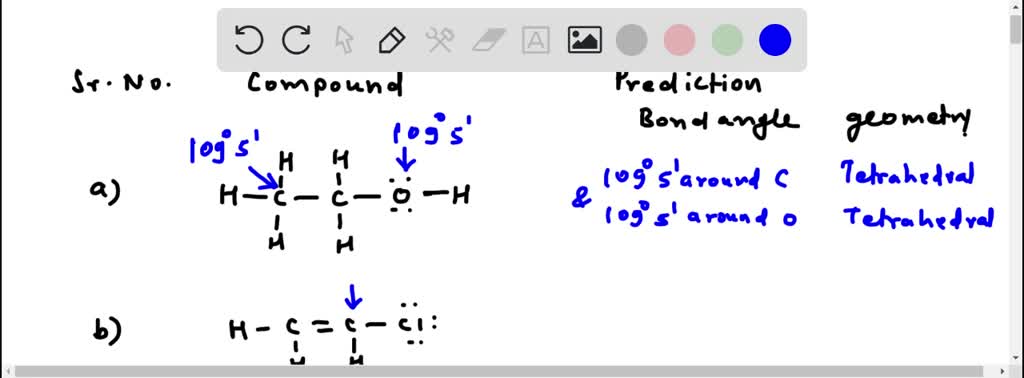
Solved:use The Vsepr Model To Predict The Bond Angles And Geometry About Each Highlighted Atom. (Hint: Remember To Take Into Account The Presence Of Unshared Pairs Of Electrons.)