Argon doesn’t want to share or exchange electrons, because the orbit at the end of argon contains electrons. A) 13 b) 9 c) 4 d) 5.

File:electron Shell 010 Neon.png - Wikimedia Commons
The atomic number of neon is z=10.
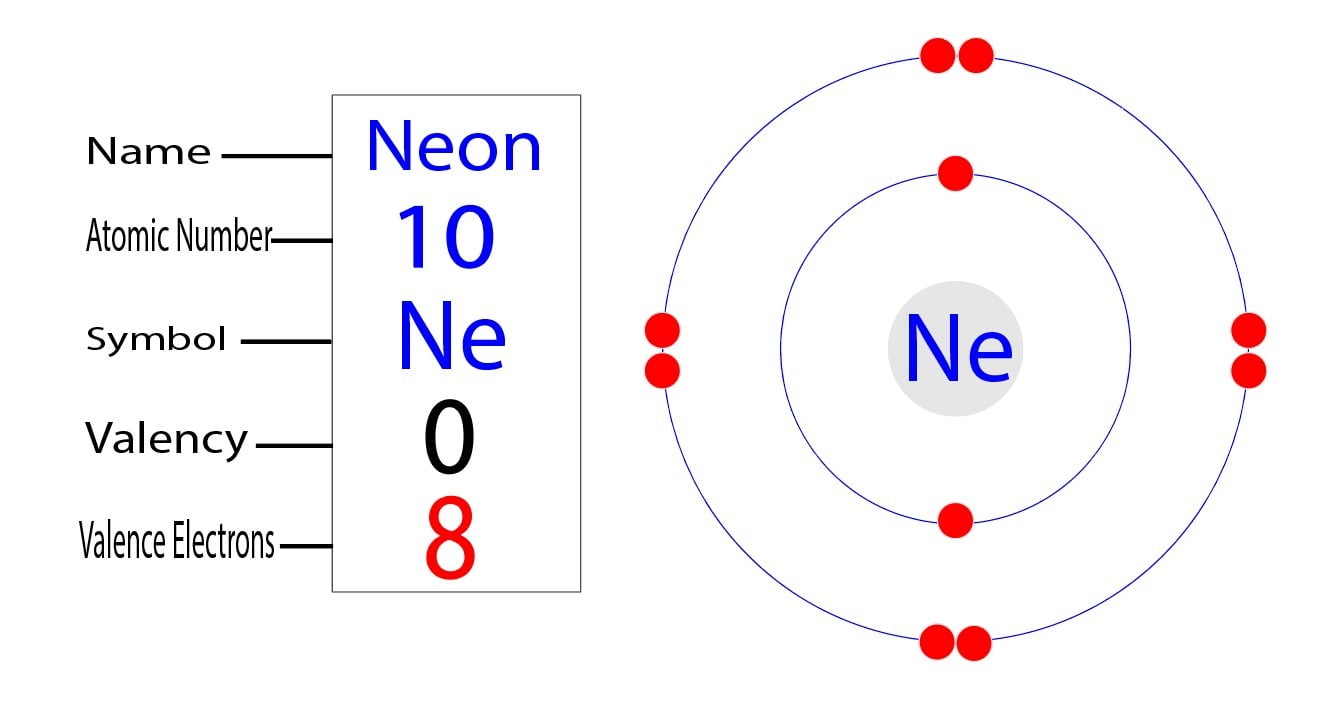
How many electrons does neon have. This means that the first shell of neon contains. It has ten electrons, which means that it has two shells. As it has 8 electrons in the outer most shell it is very stable and does not.
Valence electrons are the negatively charged particles that orbit the outermost energy shell. The least stable is 15 ne 20 ne, 21 ne, and 22 ne.
The electron configuration for neon (ne), shows that there are eight electrons in the l shell, and two in the k shell. 1 neon (ne) has an atomic number of 10 on the periodic table of elements. 1 neon, ne, has eight valence electrons, which is the reason it is a noble gas.
Enter your answer in the provided box. Enter your answer in the provided box. It is known that all neon atoms contain a total number of ten electrons.
Beryllium, be, has an atomic number of 4 and an atomic mass of approximately 9. How many are valence electrons? Neon is a chemical element with atomic number 10 which means there are 10 protons and 10 electrons in the atomic structure.
The n= 2 shell can be occupied by 8 electrons and in case of neon, all the available electronic states are occupied, i.e. The first shell can only hold two. Each neon atom has 10 protons.
So the number of electrons should also be 10. Neon, z=10 , has eight valence electrons. 10 facts about element no.
How many electrons does neon have? The inertness, the lack of reactivity, of this noble gas, is a function of its electronic configuration. Solution verified by toppr to which group of the periodic table does neon belong?
All others are under a minute, most under a second. So when you are asked How many electrons does neon have in its first shell?
The electronic configuration of a neon atom is: Neon has a closed shell configuration, and. An isotope of neon is a specific type of neon.
Implying that neon has 10 protons and 10 electrons. This means that it has the maximum number of valence electrons. Its atomic number is 10.
9 + 1 = 10 electrons find the number of neutrons The chemical symbol for neon is ne. The electron configuration of argon shows that the orbit at argon’s end is full of electrons.
However, the number of neutrons can vary depending on the isotope. How many neutrons does a beryllium atom have? Because it has a stable octet for its outer electron shell, neon atoms have 10 electrons and no net electrical charge.
Valence electrons are those that are shared between atoms. They are responsible for holding together the nucleus of an atom. A) 20 b) 10 c) 8 d) 2.
41 rows neon (10 ne) possesses three stable isotopes:

Valence Electron: Definition, Configuration & Example - Video & Lesson Transcript | Study.com