Custom art for custom needs Which of the following intermolecular forces will exist in the system?
Why hexane boiling point is low?
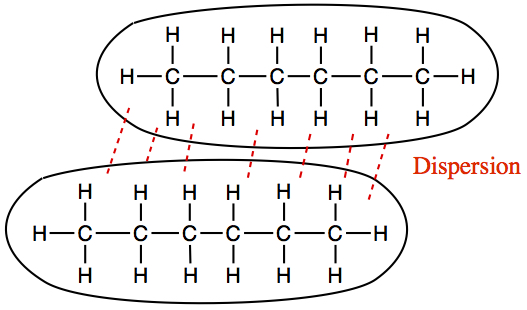
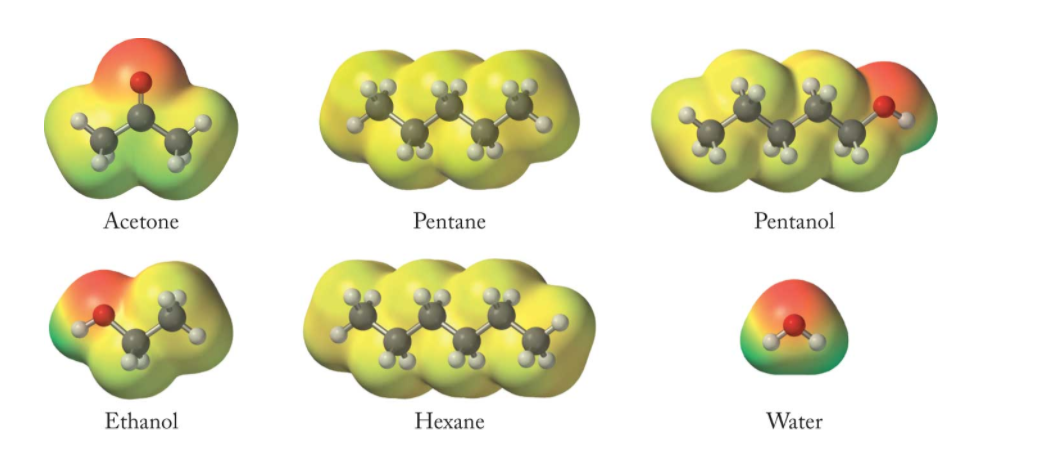
Hexane intermolecular forces. Dispersion forces are caused by the gravity created around the molecule from it taking up space, which draws in the. The influence of these attractive forces will depend on the functional groups present. The en value of carbon is 2.55 and hydrogen is 2.2.
C6h14 has very low intramolecular forces. View copy of intermolecular forces in liquids virtual lab (1).pdf from chem 101 at irvine high school. So when we're looking at the general trends for intermolecular forces, these forces are going to be cumulative.
Isopropyl alcohol has higher intermolecular forces than hexane because it. $62,000 a year is how much biweekly after taxes. This is shown in the following illustration, and since hexane is less dense than water, the hexane phase floats on the water phase.
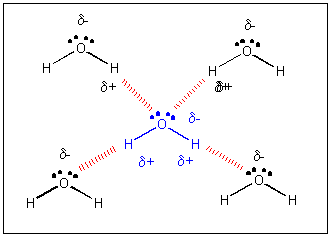
Consequently, when hexane or other nonpolar compounds are mixed with water, the strong association forces of the water network exclude the nonpolar molecules, which must then exist in a separate phase. Like most concepts in chemistry, intermolecular forces takes a bit of imagination and critical thinking to fully comprehend and apply when explaining a variety of situations. Citadel track and field schedule 2022;
Off the top of my head, london dispersion forces/van der waals forces would be the strongest interaction between hexane and iodine. What is the predominate intermolecular force between hexane (c6h14) and ethanol (ch3ch2oh)? Types of intermolecular forces :
Based on the phrase like dissolves like and the structures shown in the video, which of the solutes would you expect to be soluble in water? These forces are called intermolecular forces. What type of bonding does hexane have?
The boiling point of any compound is determined by how much energy it takes to break apart the intermolecular bonds. Intermolecular forces are generally much weaker than covalent bonds. 3/4/2021 intermolecular forces in liquids virtual.
The intermolecular forces between hexane molecules will be dispersion forces. Intermolecular forces make one molecule or ion attract another. Explain the types of intermolecular forces on hexane?