Molecular formula = c₆h₁₈o₃ empirical formula = ? What is the molecular formula?

Solved:the Molecular Formula Of Ascorbic Acid (Vitamin \MathrmC ) Is \MathrmC_6 \MathrmH_8 \MathrmO_6 . What Is Its Empirical Formula ?
This is because we can divide each number in c 6 h 12 o 6 by 6 to make a simpler whole.

Empirical formula of c6h8o6. For example, the molecular formula of glucose is c 6 h 12 o 6 but the empirical formula is ch 2 o. Visit byju s to understand the properties, structure and uses of c6h8o6 (ascorbic acid) explained by india s. The molecular formula c6h8o6 contain 20 atoms.
Give the empirical formula of: The molecular formula for vitamin c is c6h8o6. Every carbohydrate, be it simple or complex, has an empirical formula ch2o
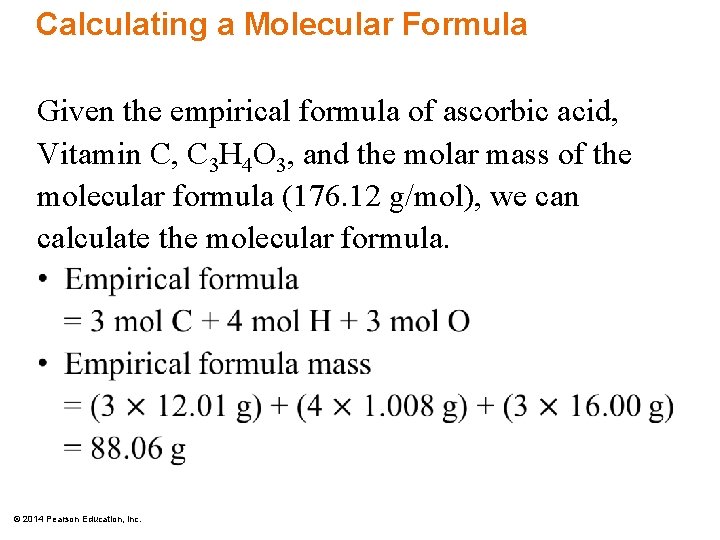
The empirical formula of glucose is ch2o. Multiply the empirical formula by this '2' to get. C3h4o3 is empirical formula of c6h8o6.
Solution for determine the empirical formula of vitamin c (c6h8o6): This means that there is one atom of carbon, 2 atoms of hydrogen and 1 atom of oxygen is. The ratios hold true on the molar level as well.
C3h4o3 is empirical formula of c6h8o6. This is the basis of the empirical formula of ethane, which is eq\rm ch_3 /eq and is different from the compound's molecular formula eq\rm c_2h_6 /eq. Thus, h 2 o is.
The molecular formula of the compound is c 6 h 6. What does c6h8o6 stand for? Calculate the molar mass of c6h8o6 in grams per mole or search for a chemical formula or substance.
An empirical formula tells us the relative ratios of different atoms in a compound. If you divide all three numbers by 2 you will get. C6h12o6 is the formula of glucose.
What would be the smallest whole number ratio between these numbers? C3h4o3 is empirical formula of c6h8o6 how many atoms does c6h8o6? Click here👆to get an answer to your question ️ fill in the blanks :empirical formula of c6h6 is.
Empirical formula molecular mass= (3*12)+ (4*1)+ (3*16)=88 dividing molecular mass (176) by empirical formula mass (88),we get 176/88=2. In this compound, the value of ‘ n ’ is 6 that means the subscript of carbon and hydrogen are divided by the whole number 6 and we get. Empirical formula is defined as the simplest ratio of the elements that form part of a molecule.
The empirical formula for c6h12o6 is ch2o. #c_8h_10n_4o_2# we can then reduce the molecular formula to the empirical. To find the empirical formula for caffeine we begin with the molecular (true) formula.
Process to find the empirical. So, you then multiply 2 by each coefficient of the elements, so c3h4o3 becomes c6h8o6.
Chapter 7 Lecture Basic Chemistry Fourth Edition Chapter

Simplest Or Empirical Formula: Ascorbie Acid (Vitamin `C`) A White Crystalline Solid, - Youtube