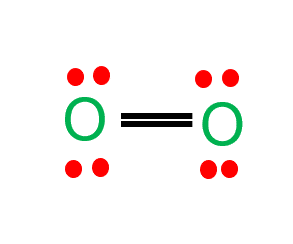
Diatomic oxygen is made up of the same two elements, and they equally share the 4 electrons that make up the double bond between them. Diatomic oxygen is made up of the same two elements, and they equally share the 4 electrons that make up the double bond between.

Is O2 Polar Or Nonpolar? - Techiescientist
Water is a polar covalent molecule.

Does o2 have polar covalent bonds. Does the oxygen molecule have a polar or nonpolar covalent bond? How do you know if a covalent bond is polar? The polarity of a bond depends on the electronegativities of the bonded atoms.
In the case of $\ce sio2 $, the structure is actually tetrahedral, with an oxygen. Bonds between carbon and other elements such as oxygen and nitrogen are polar. H2, o2 and n2 has nonpolar covalent bond.
The terms “polar” and “nonpolar” usually refer to covalent bonds. Polar covalent bonds are those where the difference in electronegativity values exists. The diatomic oxygen molecule (o2) does not have polarity in the covalent bond because of equal electronegativity, hence there is no polarity in the molecule.
Coordinate covalent bonds imply the donation of two electrons to a bond with another atom. To determine the polarity of a covalent bond using numerical. This is because of the unequal sharing of electrons (due to the difference in electronegativity) between oxygen and hydrogen.
Usually, one of the atoms involved in the covalent bond will be more electronegative and will have a greater attraction for the bonding pair of electrons. Since two atoms of the same element have the same electronegativity they. Both water and carbon dioxide have polar covalent bonds, but carbon dioxide is linear, so the partial charges on the molecule cancel each other out.
Is o2 a polar covalent bond? This gives rise to polar covalent. If covalent bond is formed between two different atoms having different electronegativity, then force acting on shared electron by the atoms.
Is o2 a polar covalent bond? Polarity is due to a difference in electronegativity.