Does nitrogen gain or lose electrons? Which elements lose and gain electrons?

Solved 1) How Many Valence Electrons Does Nitrogen Have? A) | Chegg.com
Does nitrogen gain or lose electrons.
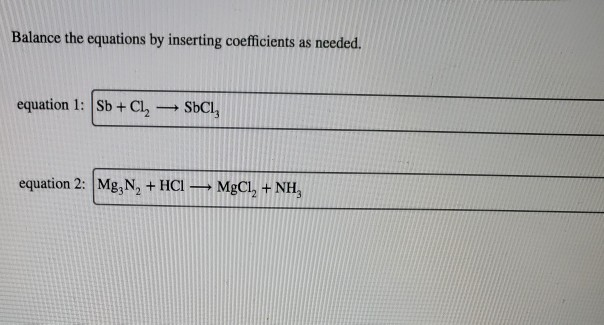
Does nitrogen gain or lose electrons. Which element would you expect to gain electrons in chemical. Metals lose electrons, nonmetals gain electrons in general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a. Elements that are metals tend to lose.
They react with other atoms with great difficulty, or not at all. 1.) n has high electronegativity (3.04) which makes it. How many electrons are gained or lost in nitrogen?
Nitrogen can also lose 5 electrons to reach stable electronic configuration of helium [1s2], but it does not lose electrons. Does nitrogen gain electrons in chemical changes? As the nitrogen element contains 5 valence electrons in the outermost shell, it needs 3 more electrons to complete its.
How many electrons are gained or lost in nitrogen? How many electrons does nitrogen lose or gain to achieve the stable octet? Does nitrogen gain or lose electrons?
Nitrogen gas does not gain or remove electrons. The element gains or loses electrons to complete its octet of 8 electrons. Complete step by step answer:
It would like to gain 3 electrons so that is will have a total of 10 making it very stable. This means that noble gas atoms neither gain nor lose electrons easily; Elements that are nonmetals tend to gain electrons and become negatively charged ions called anions.
It gains three, loses five, or shares pairs of electrons is nitrogen neutral gas? Does nitrogen gain or lose electrons in chemical changes? As the nitrogen element contains 5 valence electrons in the outermost shell, it needs 3 more electrons to complete its.
4 (368 reviews) highest rating: Typically metals we’ll lose electrons during a chemical change and form ionic bonds with non metals, the metals becoming cat ions. As the nitrogen element contains 5 valence electrons in the outermost shell, it needs 3 more electrons to complete its octet of 8 electrons.
Nitrogen is a gas and would do anything to gain electrons. Thus, a nitrogen atom will form an anion with three more electrons than protons and a charge of 3−. Does nitrogen gain or lose.
Thus it needs to combine with 4 hydrogen atoms to form a stable compound called methane (ch4). Nitrogen is the chemical element present in the periodic table in group 15 and.
How Can You Tell If An Element Wants To Gain Or Lose Electrons? | Socratic

Solved A. Ions: Transfer Of Electrons Element 2. Electron 3. | Chegg.com