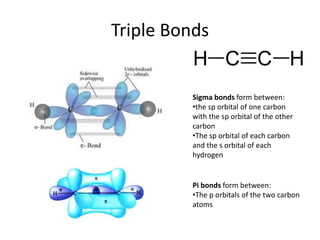
The c 2 h 2 molecule contains a triple bond between the two carbon atoms, one of which is a sigma bond, and two of which are pi bonds. Which of the following molecules contains only π bond?
46 Quantummechanicsandbondinghybridization
If u draw the structure and see it ,it

Does h2 have pi bonds. F2 is a funky in that it uses 1p bond istea of a sigma bond. Thus, each carbon atom in the ethene molecule participates in three sigma bonds and one pi bond. As the steric number of h2s is four, it has two.
Answered dec 24, 2018 by ranik (67.5k points) selected dec 24, 2018 by faiz. This condition is illustrated below. Ethene, sp2 hybridization with a pi bond the lewis structure of the molecule ch 2 ch 2 is below.
In the molecule of nitrogen (n2), for example, the triple bond between the two. Mo theory shows that there are two sets of paired electrons in a degenerate pi bonding set of orbitals. So it has to resort to a pi bond.
All halogens are single sigma bonded diatomic molecules. Thus sn of h2s molecule = 2+2. Hybrid orbitals and two lone pairs of electrons that make it an sp3 hybridization.
Pi bonds are formed from the overlap of parallel p orbitals on adjacent atoms. All the bonds present in the ethane (c2h 6) are single bonds because it is a saturated hydrocarbon. Share it on facebook twitter email.
I didn't really mean to write diatomic ( original post by nathan king) This type of overlapping can be observed in ammonia. You would surely think a triple bond would be stronger than a single bond, not weaker?
Bonding in ethyne (c 2 h 2) ethyne is the simplest alkyne in which each carbon atom is singly bonded to one hydrogen atom and triply bonded to the other carbon atom. Some such molecules are b 2 and c 2. No, water does not have a pi bond.
Pi bond, in chemistry, a cohesive interaction between two atoms and a pair of electrons that occupy an orbital located in two regions roughly parallel to the line determined by the two atoms. If a bond between two atoms is broken when one atom is rotated around the bond axis, that bond is called a pi bond. If the number of electrons in bonding orbitals is greater than the antibonding orbital, the molecule is stable.
All single bonds are sigma bonds. This is due to the small size of f. A b 2 b c 2 c c 2h 2 d both (1) & (2) medium solution verified by toppr correct option is d) there can be exclusive pi bonds without existing sigma bonds.
The bmo contains 2 electrons with opposite spins, and abmo is empty. Even if he is comparing p 2 with h 2 it's not really an answer to say it's because p 4 is more stable than p 2. They are not formed from hybrid orbitals.
Now that we know the lewis structure and hybridization of the molecule, it is easy to. Thus, h2 is a stable molecule. (a) so 2 (b) no 2 (c) co 2 (d) h 2 o.
It wants to create a sigma bond but there is too much repulsion at the distance it needs to be. One pi bond is above and below the line of the molecule as shown, while the other is in front of and behind the page. In an alkene with two carbon atoms being joined, there would be one double bond between the carbon atoms, and 4 single bonds for the 4 hydrogens, and it would be drawn as follows:
The molecular formula of water is h2o in which 2 hydrogen is linked with oxygen. Organic chemistry 1 answer ujjwal mar 22, 2018 there are no pi bonds in c2h 6. This electron pair forms the pi bond.
Which one of the following molecules contains no pi bond? The 2p z electrons of the carbon atoms now form a pi bond with each other. Since hydrogen has only one electron, it can make only sigma bond with oxygen.
A pair of atoms may be connected by one or by two pi bonds only if a sigma bond also exists between them; In general, single bonds between atoms are always sigma bonds.

Pi Bond - Definition, Explanation, Examples With Illustrations
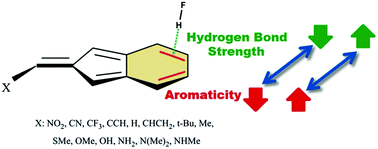
Π-Hydrogen Bonding And Aromaticity: A Systematic Interplay Study - Physical Chemistry Chemical Physics (Rsc Publishing)