School comsats institute of information. The nucleophilic site of the nucleophile is the region of a molecule that is reactive and has the electron density.

Dimethyl Sulfoxide - Wikipedia
Ch3o− or ch3oh in h2o… a:
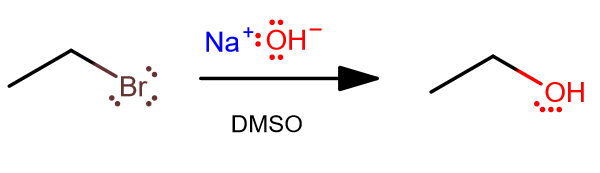
Dmso strong nucleophile. Dmf and dmso will make the ether and water layers mix. A nucleophile b electrophile c free radical d. This problem has been solved!
Resonable strong nucleophile dmf acetonitrile dmso a ow b nh 40 maleic acid is. My notes from school say that halogens* are strong nucleophiles, but weak bases and undergo sn2 rxns. Resonable strong nucleophile dmf acetonitrile dmso a.
Ch3o− or ch3oh in h2o d. Br− or cl− in dmso c. Br− or cl− in h2o b.
The choice of ethanol as solvent likely has much more to do with getting everything. Some charged nucleophiles are actually poor bases. For the legitimate reason, c 2 h 5 o − is a stronger nucleophile because the c 2 h 5 ethyl group is an electron donating group, where the electron density of the two c atoms contributes and.
A strong nucleophile is an electron rich species that is more capable of donating a pair of electrons to an electron deficient species (an electrophile), forming a dative covalent bond. In section 6.5, we learnt what makes a nucleophile strong (reactive) or weak (unreactive). Strong nucleophiles are very important throughout organic chemistry, but.
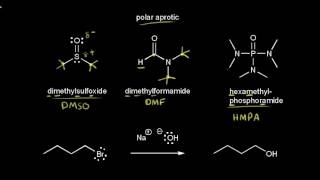
Dmso or thf (solvent) (2. Polar aprotic solvent like acetone favors sn2 reaction. Mike christensen from dbc states that halogens* are weak nucleophiles and undergo sn1 rxns;
Which is a better nucleophile?a. Im studying for the dat and have come across conflicting notes. So they often a big hassle to work with.
Methoxide dissolved in methanol vs. Br− or cl− in dmso c. Dmso solvent sn1 or sn2.
A good nucleophile, then, is not as basic and is more likely to be sterically unhindered. The freer the nucleophile, the greater its nucleophilicity polar aprotic solvents (e.g., dmso, acetone, acetonitrile, dmf) are very effective in solvating cations, but. The basicity of a nucleophile is important when you want to favor s n 2 on a hindered alkyl.
If dmso is the solvent, which would be the stronger. I searched online and can't really find a.