42 terms · solid → particles vibrate in place; Here is a brief video explaining the conversion.
The official iupac unit for gas density is kg/m 3 (not g/l).

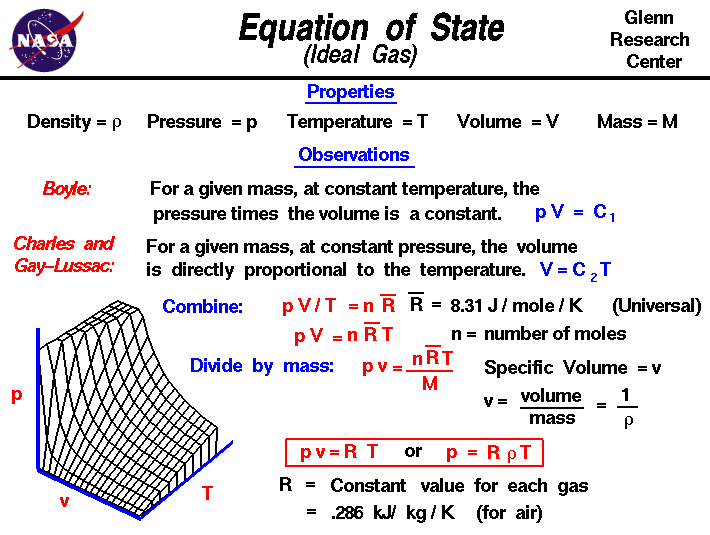
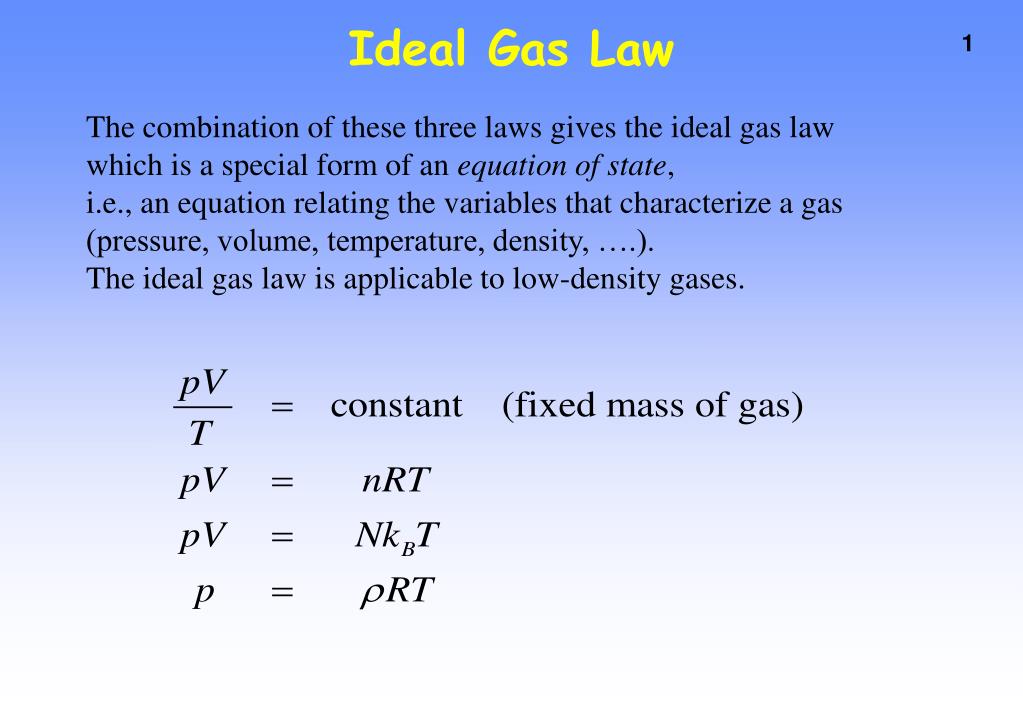
Density and gas laws. The original ideal gas law uses the formula pv = nrt, the density version of the ideal gas law is pm = drt, where p is pressure measured in atmospheres (atm), t is temperature measured in kelvin (k), r is the ideal gas law constant 0.0821 atm (l)mol (k) just as in the original formula, but m is now the molar mass ( gmol. Answer in units of pa. P v = m r t (4) where p = absolute pressure [n/m 2 ], [lb/ft 2] v = volume [m 3 ], [ft 3] m = mass [kg], [ slugs] r = individual gas constant [j/kg k], [ft lb/slugs o r]
The partial pressure of gas a is 1.53 atm; The ideal gas law can also be used to determine the density of gases. The density of gases have been listed below in alphabetical order in the units of both metric and imperial.
The original ideal gas law uses the formula pv = nrt, the density version of the ideal gas law is pm = drt, where p is pressure measured in atmospheres (atm), t is temperature measured in kelvin (k), r is the ideal gas law constant 0.0821 atm(l) mol(k) just as in the original formula, but m is now the molar mass ( g mol) and d is the density ( g. If you know the identity of the gas, you. However, it turns out that one kg/m 3 equals one g/l.
We can introduce the density of the gas into the equation by making use of the fact that molecular weight (mw) has units of mass/mole, and thus that n = m/mw, and that the ideal gas law can be written as: The ideal gas law is a good approximation of the behavior of real gases. The chemical formula as well as molar mass has also been listed.
Boyle’s law says at constant temperature, the volume and pressure of a sample of gas are inversely proportional [v % 1/p]. D = m v assume that you have exactly 1 mol of a gas. The ideal gas law can be expressed with the individual gas constant.
Here is a brief video explaining the conversion. Below the table is an image version for offline viewing. What is the formula for density?
The ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas.it is a good approximation of the behavior of many gases under many conditions, although it has several limitations. Students believe that p is always inversely proportional to v when this only works when n and t are constant. However, it turns out that one kg/m 3 equals one g/l.
The density of gas by ideal gas law formula is defined as the direct relation with pressure and molar mass of the gas and inverse relationship with the temperature of the gas and is represented as ρgas = (pgas*m)/ ([r]*tg) or density of gas = (pressure of gas*molar mass)/ ([r]*temperature of gas). Density, recall, is defined as the mass of a substance divided by its volume: That of gas b 3.8 atm.
The official iupac unit for gas density is kg/m 3 (not g/l). Charles law says at constant pressure, the volume Start studying density and gas laws.
One place teachers like to bring gas density into play is when you calculate a molar mass of a gas using pv = nrt. If we remember back to the beginning of the term the first relationship we learned was that of mass and volume to calculate the density of different materials. Students use the ideal gas law assuming that one variable is either directly or inversely proportional to another without considering that this only works if all other variables are constant.
Ρ = pm/rt, where m is molar mass. 23 rows jul 26, 2015. Noting that m/v is density, ρ, the equation can be written as p (mw) = (m/v)rt = ρrt.
Endot…, freezing → liquid changes to solid; The ideal gas law, molar mass, and density there are several relationships between the temperature, pressure, the number of moles and the volume of gases. What is the final pressure?
One place teachers like to bring gas density into play is when you calculate a molar mass of a gas using pv = nrt. A fluorinated organic gas in a cylinder is compressed from an initial volume of 910 ml at 150 pa to 492 ml at the same temperature. Gas laws and density calculations:
Learn vocabulary, terms, and more with flashcards, games, and other study tools. A mixture of three gases, a, b, and c, is at a total pressure of 7.04 atm. Calculating the density of a gas usually involves combining the formula for density (mass divided by volume) and the ideal gas law (pv = nrt).
Cl…, liquid → molecules are still pretty clo…, gas → particles are fast and can bre…, plasma → particles are broken into ions…, melting → solid changes to liquid;
Ppt - Ideal Gas Law Powerpoint Presentation, Free Download - Id:6624513