We should know that the conjugate acid of a weak. What is the conjugate base of h2po4?
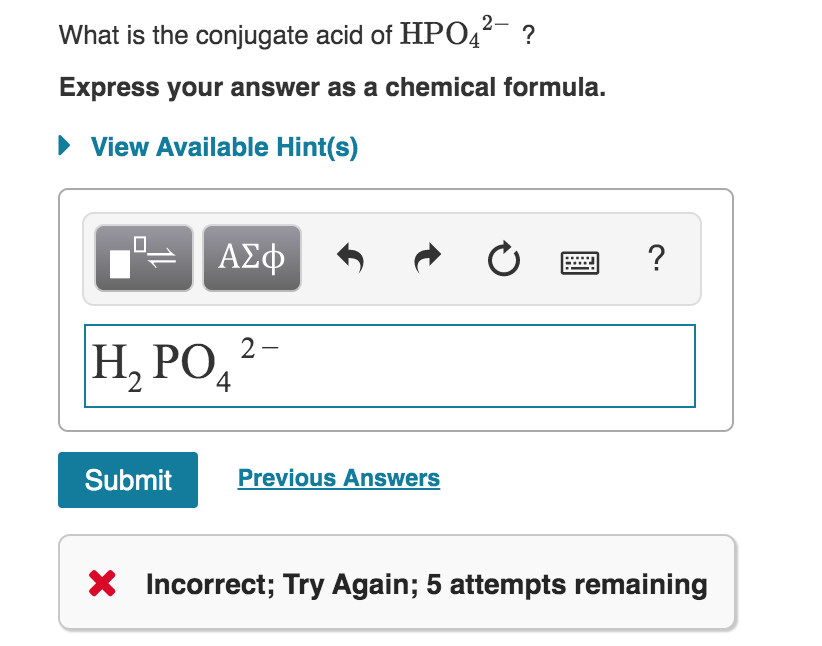
Solved What Is The Conjugate Acid Of Hpo42- ? Express Your | Chegg.com
If it’s already in water, it will further dissociate.
Conjugate acid of hpo4-2. It is the conjugate base of phosphoric acid h3po4 and the conjugate acid of monohydrogen. Or rather phosphoric acid donates a. The conjugate acid of hpo4 2 is h2po4 o true o false.
The conjugate acid of is: Phosphoric acid is the parent acid, i.e. The one hydrogen atom is attached to one of the oxygen atoms with a single covalent bond.
See the answer see the. H 2po 4− d po 43− medium solution verified by toppr correct option is c) the conjugate acid of hpo 42− is h 2po 4− h 2po 4−→h ++hpo 42− the base and its conjugate acid differ. The conjugate acid of hpo4 2 is h2po4 o true o false ;
What best describes a bronsted lowry acid base reaction? H p o 4 − 2 + h + → h 2 p o 4 −. So the correct answer is option c.
Therefore, the base hydrogen phosphate ion reacts with a proton to form a conjugate acid in the following manner: The resulting base is called the conjugate base. What is the conjugated acid of h2po4?
It has to be an acid in molecular form. You cannot put as it is in water. Conjugate acid is h2so4 conjugate base is so42.
Hence, the conjugate acid of h p o 4 −. This problem has been solved! The conjugate base and conjugate acid for hs04 is:
Remove a proton from this, we get, h 2p o− 4 as the conjugate base. This is the best answer based on feedback and ratings. Therefore, the conjugate acid that is formed is h 2 p o 4 −, which is dihydrogen phosphate ion.
The three oxygen atoms are connected to the phosphorus atom with single covalent bonds. By continuing to use this site. Join / login >> class 11 >> chemistry >> equilibrium >> acids, bases and.

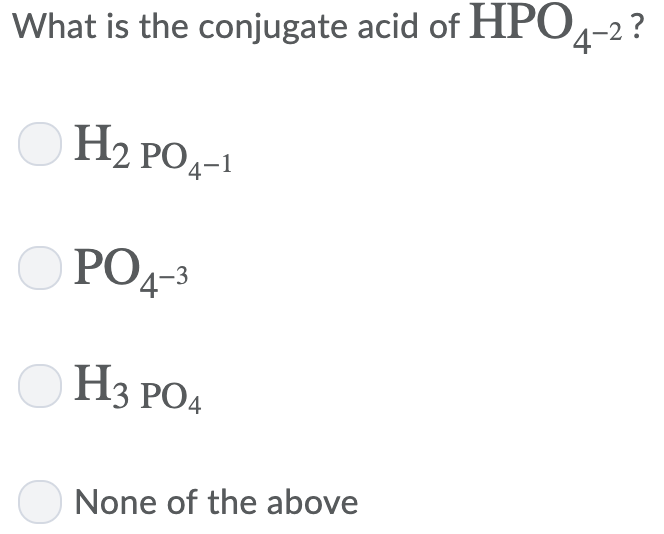
Solved:question 27 What Is The Conjugate Acid Of Hpoa ? 0 Hapoa 0 Hso" 0 Hpo4? 0 0L1
