Use the diagram in the transition to determine the order of orbital filling. A) 1522s22p63s23p64s24p6 b) 1s22s22p63s23p64s24d104p6 c) 1s22s22p63s23p64s23d104p6 d).

Electron Configuration For Argon (Ar)
Since 1s can only hold two.

Complete the electron configuration for as. The complete electron for a neutral arsenic atom is: The condensed electron configuration for (a) co (element 27), (b) in (element 49) has to be written. Electron configuration of oxygen chlorine electronic configuration chlorine.
The electron configuration of all the elements can be done through orbital diagrams. In a neutral atom, the number of. If add this, we get 2 + 4 = 6.
Electron configuration for fe, fe2+, and fe3+ (iron and iron ions) in writing the electron configuration for iron the first two electrons will go in the 1s orbital. 119 rows the electronic configuration of each element is decided by the aufbau principle which states that the electrons fill orbitals in order of increasing energy levels. Once you practice a few different examples writing.
To write a complete electron configuration for an uncharged atom, determine the number of electrons in the atom from its atomic number. In order to write the as electron configuration we first need to know the number of electrons for the asatom (there are 33 electrons). When we write the configuration, we'll put all.
119 rows electron configuration chart of all elements is mentioned in the table. The electron configuration of arsenic, asas, is 1s22s22p63s23p64s23d104p31s22s22p63s23p64s23d104p3. Sulfur electron configuration is, 1s2, 2s2, 2p6, 3s2, 3p4 the highest energy level in electrons configuration is, 3s2 3p4.
119 rows use the element blocks of the periodic table to find the highest electron orbital. 1) cr 2) s 3) v 4) mn 5) fe. (see below.) add electrons to the sublevels in.
As is the chemical symbol for the element arsenic. Solution for write the complete electron configuration for following atoms: Therefore, the electron configuration of oxygen is 1s2 2s2 2p4, as shown in the illustration provided below.
Enter the complete electron configuration for cadmium, cd, which has atomic number 48. The complete electron configurations concept builder challenges learners to recognize the electron configuration for the atoms and the ions of various elements. This video explains how to use the aufbau principle and a diagonal diagram to write electron configurations.
So, the valency is 6. Write the electron structure of the +1 cation of thallium. 14 write the electron configurations for the following atoms or ions:
1 s 2 2 s 2 2 p 6 3 s s 2 2 s 2 2 p 6 3 s Alternatively, remember group 1 (alkali metals) and group 2 (alkaline earth metals) are. Its atomic number is 33, which is the number of protons in the nuclei of its atoms.
When we write the configuration we'll put all 18 electrons. In order to write the argon electron configuration we first need to know the number of electrons for the ar atom (there are 18 electrons).
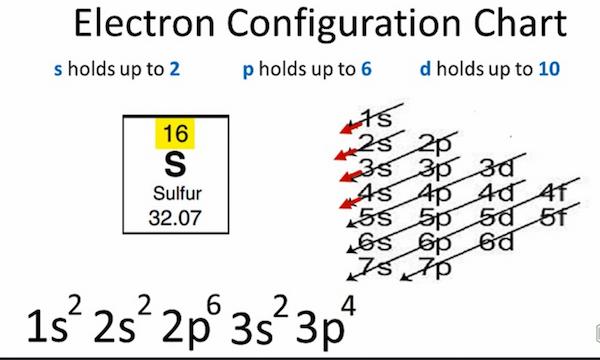
Sulfur Electron Configuration And Molar Mass - What's Insight