1 bond to another atomor lone pair = s (not really hybridized) 2 bonds to another atomor lone pairs = sp 3 bonds to another atomor. The number of lone pairs on a given atom can be calculated by using following formula.
How Many Pi Bonds Are There In "Co"_2? | Socratic
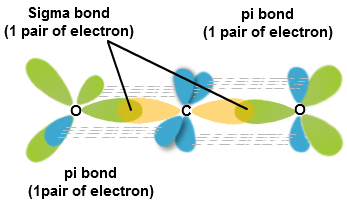
The central atom c is sp hybridized here and there are two sigma bonds and two π bonds are present between c and o atoms.
Co2 hybridization number of sigma bonds. A faster way to determine how many pi bonds the molecule has is to know that a double bond is comprised of 1 sigma and 1. Therefore, co2 has 2 pi. The following rules give the hybridization of the central atom:
Hybridization of co2 = ½ [ 2+2+0] = 2 = sp co2 molar mass molar mass (m) of any molecule is defined as the total sum of the mass of each atom in the molecules in grams per. In sulfur hexafluoride sf6 s f 6, sp3d2 s p 3 d 2 hybridization is seen where six sigma bonds are present around the sulfur atom and no pi bonds are present. The number of sigma bonds formed by sulfur atom is two since it is bonded to only two oxygen atoms.
Therefore, the total number of. The atomic number of the carbon is six which makes its electronic configuration 1s2 2s2 2p2. Its px orbital will form a pi bond with the o atom that has its px orbital unhybridized, while its pz orbital will for a pi bond with the other o atom's pz orbital.
Sigma bonds from sp and sp2hybrid orbitals sp2hybrid orbitals in borane atoms that have 3 bonds, 2 bonds and 1 lone pair, or 1 bond and 2 lone pairs need 3 orbitals that are 120 degrees. Here in the cs2 molecule, the number of sigma bonds on the central atom is two, and there are no lone pairs on the central atom as its octet is complete by sharing the valence. Both the p y and the p z orbitals on each carbon atom form pi bonds.
It can be easily seen that the only type of covalent bonds present in alkanes are sigma bonds, also loosely known as single bonds. Once that is done move to the next step. Therefore, co2 has 2 pi bonds and 2 sigma bonds.
Second, count the number of covalent bond that. The sp hybrid orbitals form a sigma bond between each other as well as sigma bonds to the hydrogen atoms. Calculate the number of sigma (σ) bonds.
All the atoms of co2 molecule lie in the same plane. Sn = number of lone. First, is to be able to draw the structure.
Science chemistry library chemical bonds hybridization and hybrid orbitals. How can i determine the hybridization of co2? You must first draw the lewis structure for co2.
As the 2p shell has a capacity of holding up to six electrons, there comes a deficiency. According to vsepr theory, we can use the steric number (sn) to determine the hybridization of an atom. (from ground state à hybridized state) show # of orbitals involved in.
This case arises when there are no lone pairs on the given central atom.

Hybridisation And Bonding In Carbon Dioxide - Youtube

Molecular Orbital Theory - Π Bonding In Carbon Dioxide - Chemistry Stack Exchange