(a) 25,0 g naoh (d) 14.8 g ch2oh (b) 44.0 g br2 (e) 2.88 g na2so4 (c) 0.684 g mgcl2 (f) 4.20 lb zni2 this problem has been. Click here 👆 to get an answer to your question ️ how many moles of kbro3 are required to prepare 0.0700.
Ppt - Ch 9 Section 1 Review Page 311 #4-5 Powerpoint Presentation, Free Download - Id:3781182
The small number after the element symbol is called the.

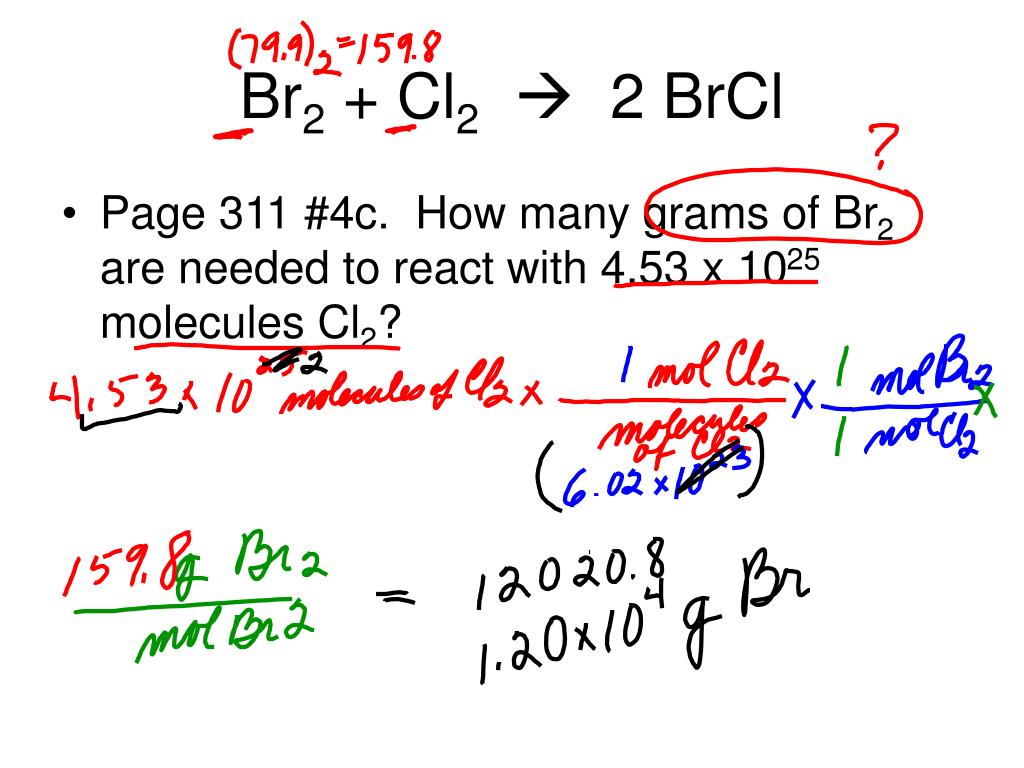
Br2 how many moles. When calculating molecular weight of a chemical compound, it tells us how many grams are in one. 2 (109/159.8) of br2 = 2/3 x ( 109/159.8)x26.98 al = 12.27g. When you write mol instead of mole.
44.0 grams br2 (1 mole br2/159.8 grams)(6.022 x 10^23/1 mole br2)(1 mole br2 atoms/6.022 x 10^23) = 0.275 moles of br2 atoms To find the total number of atoms in br2 (bromine gas) we’ll add up the number of each type of atom. How many moles of atoms are contained in the following?
Convert grams br2 to moles or moles br2 to grams. What is the molar mass of. We assume you are converting between moles br2 and gram.
You can view more details on each measurement. Most noteworthy, each molecule has 1 na (sodium) and 1 cl. N = 102/119.002 n =.
You can express the above. .09.2020 chemistry secondary school answered • expert. Comparing the moles ratio of br2 to al is 3 :
Struggling with understanding significant discoveries involving atom. How many moles of br2 are in five cubic meters (5.00 m3) of br2 gas at stp? Moles of kbr in 102 g of potassium bromide.
How many moles br2 in 1 grams? Molar mass, molecular weight and elemental composition calculator molar mass of br2 is 159.8080 g/mol compound name is bromine get control of 2022! Moles = mass/molar mass and = moles x molar mass.
26 upvotes · 3 comments. 1 mole br2 = 159.808g br2 = 6.022 x 1023 molecules br2 4.89 x 1020 molecules br2 x 1mol br2/6.022 x 1023 molecules br2 x 159.808g br2/mol br2 = 0.130g br2. How many moles of br2 are in two cubic meters (2.00 m3) of bra gas at stp?
From the balanced chemical equation, we can say that 1 moles of kbr will produce 1 moles of kcl. Moles kcl = 4.3 * 2 / 1 = 8.6 moles kcl hope this helps! What is the molar mass of bromine br?
Use ideal gas equation to solve for npv = nrt n = pvrt given that,volume, v = 5 m3 = 5000 l 1m3 =… 29 upvotes · 1 comments. What is the mole of br2?
What is the molar mass of br2. 100% (6 ratings) transcribed image text: A few things to consider when finding the molar mass for br2:
Solved How Many Moles Of Bromine, Br2(1), Are There In 10.0 | Chegg.com
Molar Mass / Molecular Weight Of Br2 : Bromine Gas - Youtube