The chemical symbol for boron is b. If you look in the periodic table, you will notice that boron has 5.

Boron Stock Illustration. Illustration Of Symbol, Electrons - 89682672
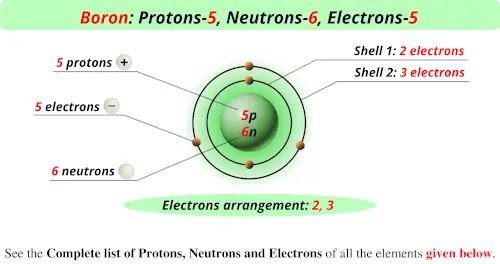
As, the number of protons and electrons are equal in a neutral atom, therefore, number of electrons and number of protons in boron is 5.

Boron 11 protons neutrons electrons. How many protons and neutrons. The number 11 represents the mass number which is the sum of protons and neutrons. How many protons neutrons and electrons are in boron 11?
Boron has 5 protons, 6 neutrons, and 5 electrons. That is, the charge of an electron is equal to that. The number 11 represents the mass number which is the sum of protons and neutrons.
If a boron atom has 5 neutrons in it’s nucleus to accompany the 5. If you look in the periodic table, you will notice that boron has 5 protons. Then the mass number is total protons plus neutrons.
The atomic mass of boron is 10.811, so we’ll take the roundup value as 11. The atomic number of boron is 5, meaning there are 5 protons in all isotopes of boron. Protons and neutrons are located in the nucleus.
How many electrons, protons and neutrons does a boron atom have? The electrons account for the chemical behaviour of boron, and this chemical behaviour provides a means of identifying it. Boron is a chemical element with atomic number 5 which means there are 5 protons and 5 electrons in the atomic structure.
Then the mass number is total protons plus neutrons. The nucleus is located in the center of the atom.
Chemical Elements.com - Boron (B)
Protons Neutrons & Electrons Of All Elements (List + Images)