Their conductivities are two orders of magnitudes less than most metals. Log in for more information.

Properties Of Metals, Nonmetals, And Metalloids - Engineering Choice
Metalloids can be malleable and ductile, like metals.

Are metalloids good conductors. Metals are good conductors because they form metallic bonds and metallic bonds allow for the flow of electrons, which is electricity. What are metalloids good for? Metalloids are usually goodish conductors as well (they're actually semiconductors).
The capacity to conduct electrical charge and heat. It is not easy to distinguish them from true metals. Aliciadmanrajos aliciadmanrajos 05/23/2017 biology high school answered metalloids are good conductors of electricity.
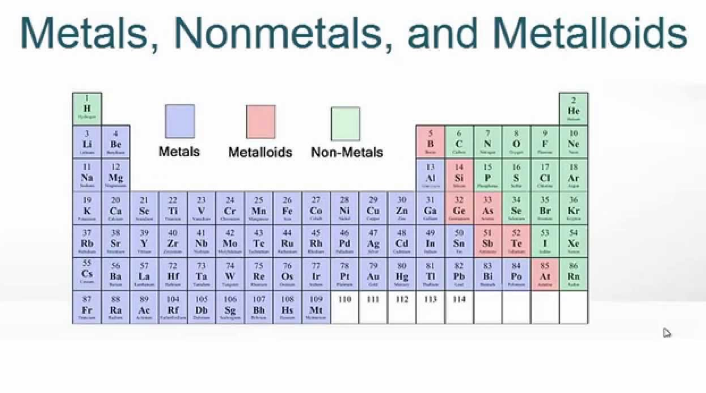
A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Whether or not a metalloid conducts electricity can depend on the temperature or the exposure to light. Most of the metals are solids at room temperature, with a characteristic silvery shine (except for mercury, which is a liquid).
Most metals are excellent conductors. Metalloids are good conductors of electricity. Is metalloid a good insulator?
Find an answer to your question metalloids are good conductors of electricity. You will find the metals on the left side of the periodic table. Metalloids have intermediate heat and electrical conductivity.
Most are semiconductors, and moderate thermal conductors, and have structures that are more open than those of most metals. Some metalloids ( as, sb) conduct electricity like metals. More answers below apeksha chhabra
Is it true or false? The intermediate conductivity of metalloids means they tend to make good semiconductors. Most metalloids have a metallic lustre but are poor conductors of heat and electricity.
See answer (1) best answer. The elements can be classified as metals , nonmetals, or metalloids. This answer has been confirmed as correct and helpful.
It similarly allows for good conduction of heat. Metals mercury and gallium are not as good conductors as most metals (but they are good conductors). The free electrons are competent to conduct both charge and.
They also can be brittle, similar to nonmetals. Physical properties of metalloids for example, metals are good conductors of both heat and electricity, whereas nonmetals generally cannot conduct heat or electricity. For this reason metalloids, such as silicon or germanium, are used to make semiconductors.
Is it true or false? The most useful property of metalloids is their varying ability to conduct electricity. They have few electrons in their valence shells.
The metalloids, as the smallest major category of elements, are not subdivided further. But the free electrons are delocalized across the entire lattice, and thus they give rise to two other bulk properties: Note that mercury/gallium still are better conductors than graphite.
Metals are good conductors of heat and electricity, and are malleable (they can be hammered into sheets) and ductile (they can be drawn into wire).
10 Difference Between Metals, Nonmetals And Metalloids (With Examples) - Viva Differences

7.6 Metals, Nonmetals, And Metalloids - Ppt Video Online Download | Ionization Energy, Electron Affinity, Electron Configuration