29,853 you have to think about the whole process. Zinc doesn’t react with cold water as it is not reactive enough to react with cold water.

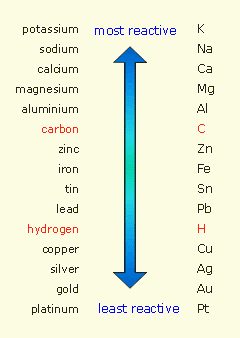
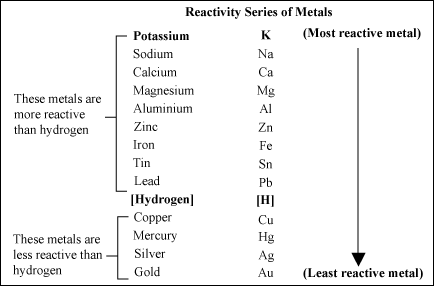
Reactivity Series Of Metals | Secondary Science 4 All
Why does zinc not react with cold water?
Why is zinc so reactive. This shows magnesium is more reactive than zinc and both metals are more reactive than hydrogen. Why is zinc less reactive than magnesium? If you look at the electron configureation of zinc, it is [ar]3d104s2 so it has two valance electrons that it readily gives up in order to form aqueous.
Copper metals are able to delocalize its outer electrons more effectively than zinc. Magnesium displaces three metals, zinc displaces two metals, iron displaces one metal and copper does not displace any of the. Why is zinc non reactive?
To make the reaction of zinc with water. Please remember, the higher the position of a metal,. This third displacement reaction can be used for any metal that.
The potassium is in the uppermost position of the table than zinc, and therefore, potassium is the most reactive of all the metals. When you add zinc or copper to hydrochloric acid, the metals potentially may be oxidized by hydrogen ions in. Why is zinc more reactive than copper?
The reason for this is due to the reduction potential of the two elements. Why is zinc less reactive? Copper’s metallic bond is therefore stronger than zinc, so more.