The combined gas law has practical applications when dealing with gases at ordinary temperatures and pressures. There is no official discoverer of combined gas law.

What Is The Difference Between Combined Gas Law And Ideal Gas Law | Kinetic Gas Theory - Youtube
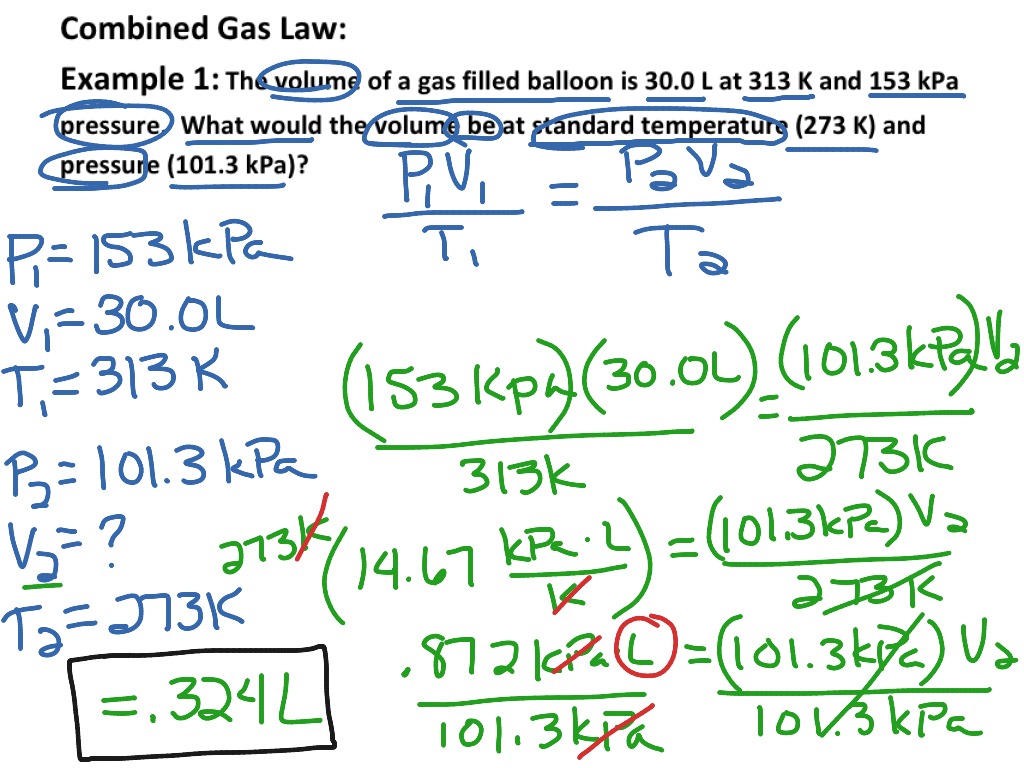
Combined gas law formula states that the product of pressure (p) and volume (v) of a given quantity of gas divided by the temperature (t) of that gas is constant.
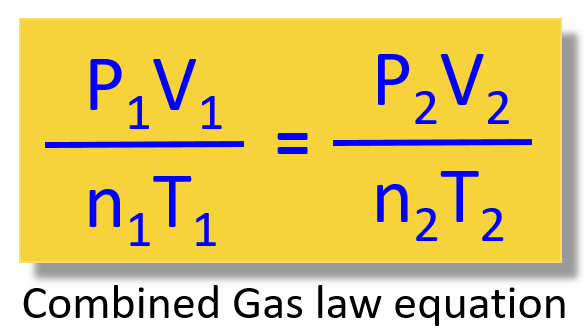
When to use combined gas law. Uses of the combined gas law the combined gas law has practical applications in situations where pressure, volume, or temperature can change. It states that the ratio of the product of pressure and volume and the. Pv/t = k in which p is pressure,.
The interdependence of these variables represents combined gas law which states that the ratio. Find the final temperature, given that the initial. The pressure of a gas is reduced to 75% of its initial value and the volume is increased by 40% of its initial value.
Apply the concept of the. The combined gas law combines the three gas laws: If you look carefully, the ideal gas law has a term in it which the combined gas law does not.
The combined gas laws indicate that the ratio of the product of pressure and volume and the absolute temperature of a gas is equal to a constant. These laws relate one thermodynamic variable to another holding everything else constant. Moreover, this law works when everything with the exception of volume,.
Given two pressures, volumes, or temperatures and asked for an unknown pressure, volume, or temp. It is used in engineering,. Oh, and only when the.
What is the combined gas law used for? The combined gas law is useful when: Combine gas law is simply a combination of the other gas laws.
So, use it when you need to deal with that term, otherwise the cgl is fine. In order to compute the changes in temperature, pressure or volume a sample gas may suffer in certain conditions, the combined gas law can be written in the form detailed within the next. Perform calculations to determine the mole fractions of gases within and gas mixture and relate mole fraction to the partial pressure of a gas within a gas mixture.