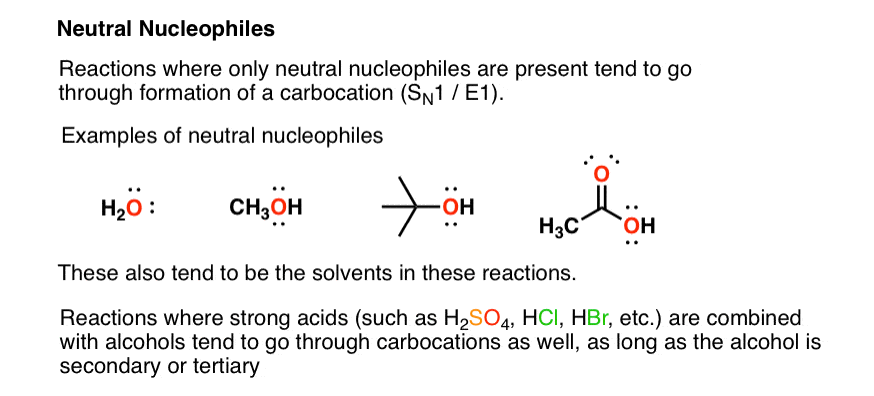
It is a common misconception that good leaving groups should be weak nucleophiles. In general, weak bases are also weak nucleophiles.
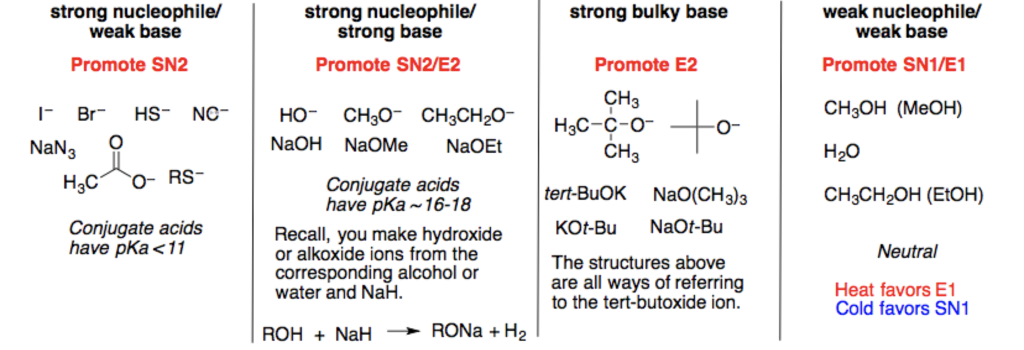
Solved Strong Nucleophile/ Weak Base Strong Nucleophile/ | Chegg.com
Strong nucleophile/ weak base strong nucleophile/ strong base weak nucleophile weak base strong bulky base promote sn2 promote sn2/e2 promote e2.
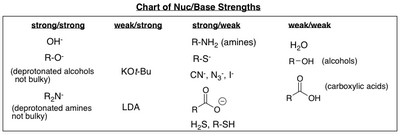
Weak bases make strong nucleophiles. They will easily donate their electrons, and take part in e1 or sn1 mechanisms with carbocation. But weak bases can also be good nucleophiles. Nucleophilicity is the strength of the species to behave prominently as a nucleophile in chemical reactions.
This happens when a substance loses its electrons (lewis base). In general, good bases are also good nucleophiles. Carboxylate ion ( r c o o x −) is a weak base as the negative charge on oxygen is delocalised due to resonance.
Learn vocabulary, terms, and more with flashcards, games, and other study tools. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Best job sites for senior executives;
However, there is a simple counterexample: Once you understand the similarities and differences between nucleophiles and bases, you need to understand how to differentiate the molecules that prefer to act as just one. Yes, a strong nucleophile can be a weak base.
With a few exceptions, a strong nucleophile is also a strong base. Polarizability is defined as the ability to distort the electron cloud of an atom, which allows it interact with a. These weak bases that are strong nucleophiles include azides, halides and thiols.
Where to shoot a coyote with a shotgun; Strong base and strong nucleophilestrong base and weak nucleophileweak base and strong nucleophileweak base and weak nucleophile Iodide is known to be both a good.
Start studying strong bases, strong nucleophiles, weak bases, weak nucleophiles. Start studying strong vs weak nucleophiles and bases. Because the hydrohalic acids are strong acids, the halide ions themselves are weak bases.
Bases turn the colour of red litmus paper to blue. So, it has less tendency to donate lone pair of electrons to h+. Weak bases, weak nucleophiles, strong bases, and strong nucleophiles.
What makes a good nucleophile? Most valuable non sports cards; All nucleophiles are brønsted bases — they donate a pair of electrons to form a bond to another atom.
Therefore, weak bases such as neutral oxygens with a proton will also be weak nucleophiles.