Six, it's 14 and 68 14. Hbr co co 2 krcl 2 xecl 2 h 2 expert answer hbr is polar molecule.
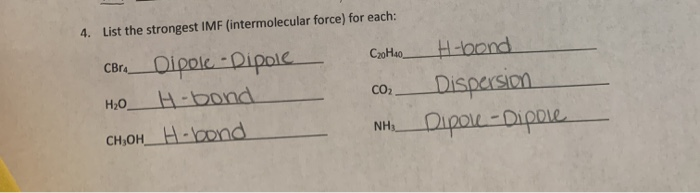
Solved 4. List The Strongest Imf (Intermolecular Force) For | Chegg.com
See the answer what is the strongest type of intermolecular force in the following compounds?
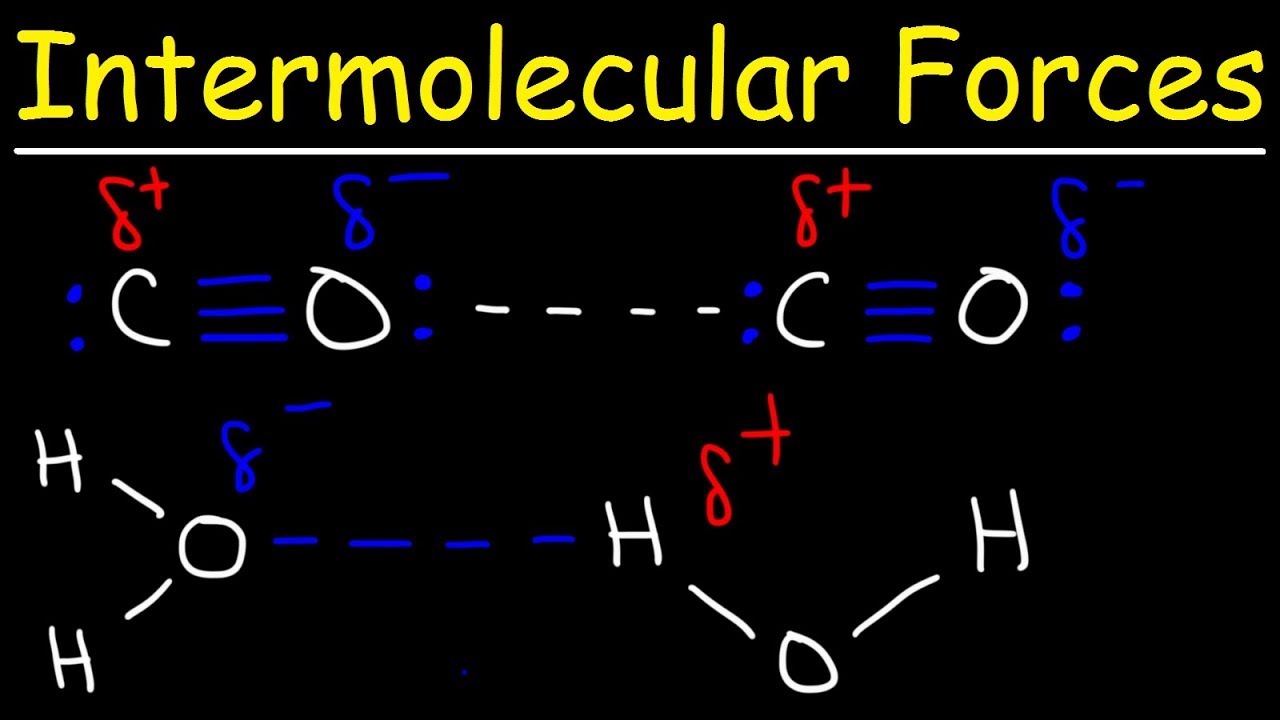
Strongest intermolecular force in co2. For these we need hydrogen atoms bonded to one of the three most electronegative atoms (n, o or f) so that the hydrogen atom has a partial positive charge, and we need a lone pair available on the electronegative atom. It means that highle electronegative oxygen attracts the low electro negative carbon towards it hence creating partial charges 1 co 2 2, or carbon dioxide, is a linear, nonpolar molecule even though the bonds (between c and o) are polar.
Thus, although co₂ has polar bonds, it is a nonpolar molecule. Let’s first look at what options we have for intermolecular force. Co2 has no h atoms, so no hydrogen bonds.
For extra information, there are 3 types of intermolecular forces. Carbondioxide has two polar c=o bonds in which the dipoles are cancelled out thereby making it a non polar compound. The only intermolecular force found in carbon dioxide is the london dispersion forces and they are forces which are caused by electron movement which forms dipoles.
Co is best example for force of attraction and repulsion between molecules. What is the strongest intermolecular force in 1 pentanol? 6 what is the strongest intermolecular force present in solid co2 ?
What is the strongest intermolecular force between co2 and co? * * no van der waals force 1. Draw the structure of co2.
The only intermolecular force that exists for this molecule is. So, we can say that, different molecules created different types of intermolecular forces such as, dipole dipole interaction, hydrogen bonding, ion dipole interaction, and london dispersion forces. Okay, so today we are going to be um talking about the strongest type of inter molecular attraction that exists in each of the following liquids.
But it all depends upon molecules and atom. Intermolecular force here will be the connection between each so2 molecule. The dipoles point in opposite directions, so they cancel each other out.
The strongest i.m.f present b/w co2 is ion induced forces. So2 is a polar molecule with dipole dipole forces. The most important factor in determining the boiling point, is the intermolecular forces involved.
Co2 has dispersion forces or van der waals forces as its only intermolecular force. Um it's going to have um yeah mostly just london version forces in the most present there because it's not a polar molecule and it doesn't have a die poll because it's distributed more or. Rank the following molecules in order of increasing strength of intermolecular forces.
What is the strongest type of intermolecular force in the following compounds? * * no ionic bond * is so2 an ion? Being a linear molecule, co2 is non polar and hence the only force acting between co2 molecules is london dispersion force which the weakest intermolecular force of attraction.
Hbr co co2 krcl2 xecl2 h2 this problem has been solved! In this video we’ll identify the intermolecular forces for co2 (carbon dioxide). Co2 is a nonpolar molecule, thus having.
H bond * is there hydrogen atom in so2? There are 3 types of intermolecular forces: The strongest type of intermolecular forces are called hydrogen bonds.
