In sp2 it is 33.33% whereas in sp3 it's just 25%. Thanks to nitrogen introduced into the layered carbon framework of graphite, the chemical reactivity of the carbon atoms was increased.
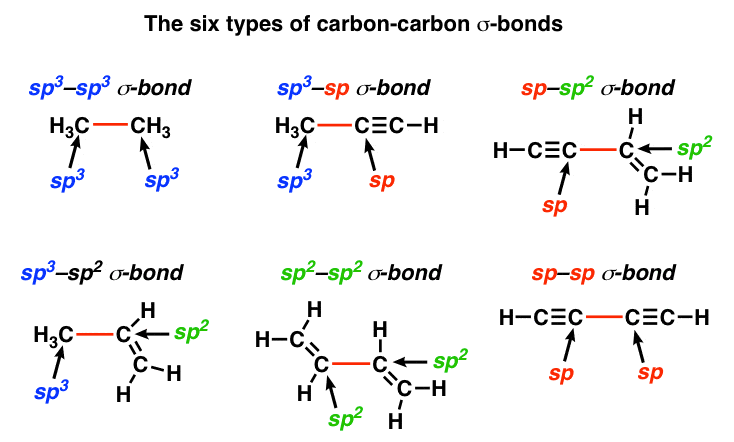
Sigma Bonds Come In Six Varieties: Pi Bonds Come In One – Master Organic Chemistry
However, the carbon in these type of carbocations is sp2 hybridized.
Sp2 hybridized carbon. An exception to the steric number. There is a formation of two single bonds and one double bond between three atoms. What is sp2 hybridised carbon?
Two sp 2 hybrids bond with the hydrogen atoms, and the other forms a sigma bond with the other carbon atom. Now an s orbital being spherical is more close to nucleus, as it is attracted from all the. The newly formed hybrid orbitals are known as sp 2 hybrid orbitals.
Carbon dots (cdots) are a promising biocompatible nanoscale source of light, yet the origin of their emission remains under debate. If you can overlap perfectly only by rotation and translation, the atom is symmetric; Here, we show that all the distinctive optical.
Sp 2 hybridization is also called trigonal hybridization. And use a different color here. In sp hybridised carbon, the %s character is 50% ;
This is an sp2 hybrid orbital and same with this one, an sp2 hybrid orbital. The major product 'a' of the following given reaction has _____ sp2 hybridized carbon atoms. We call this sp2 hybridization.
Let me go and write this up here. Since its successful experimental preparation by novoselov and geim in. It involves mixing of one ‘s’ orbital and two ‘p’ orbital’s of equal energy to give a new hybrid orbital known as sp 2.
A mixture of s and p. Sp 2 hybridization is the mixing of one s atomic orbital with two p atomic orbitals. The lewis structure for ethene the carbon atoms are sp 2 hybridized.
However, i am having a difficult time. The sp 2 hybridization is the mixing of one s and two p atomic orbitals, which involves the promotion of one electron in the s orbital to one of the 2p atomic. Since s p 2 carbon atoms.
If the two mirror images act as left and right hands, it is asymmetric.
Does Sp2 Hybridization Mean That The Atom Will Form Three Bonds (We're Talking Any Atom, Not Just Carbon)? - Quora
What Are The Possible Term Symbols For The Hybridized Carbon Atom (Sp, Sp2, Sp3)? - Quora