Concentration of hydroxide ion [oh¯] = 3.0x10¯³ m poh =? Naoh güçlü bir baz olduğu için tamamen su içinde iyonize olur.

W Arm -U P What Is The Ph Of A M Solution Of Hi? What Is The Poh Of A M Solution Of Naoh? What Is The [H+] Concentration Of A Solution
Then the ph of hcl solution having same.
Poh of naoh. Molar concentration is equal to moles of solute per liter of solution: Solution verified by toppr correct option is d) the hydroxide ion concentration in neutral water is 10 −7. This will produce a ph of 13.
The hydroxide ion concentration in 10 −8 m naoh solution will be 10 −7+10 −8= 10 −7×1.1 poh=−log[h +]=−log10 −7×1.1=7−log1.1=6.96. Now you apply poh+ph =14 (at 25 degree centigrade) to calculate ph of 0.5 m naoh. 100% (1 rating) transcribed image text:
Now you apply poh+ph =14 (at 25 degree centigrade) to calculate ph of 0.5 m naoh. Of naoh is 0.5 , from this you can calculate poh. 0 0 similar questions the ph value of ordinary water is:
Calculate the ph and the poh of each of the following solutions at 25∘c for which the substances ionize completely: You are given that the ph = 4.5. 1 chemical foundations 2 atoms, molecules, and ions 3 stoichiometry 4 types of chemical reactions and solution stoichiometry 5 gases 6 thermochemistry 7 atomic structure and periodicity 8.
Since ph + poh = 14 we can calculate the ph to be 13. 1 mole of naoh produced 1 mole of oh¯. 4 answers #2 for a were told about the h three.
This can be obtained as illustrated below: Oh plus similarity is equal to 5.3 times 10 to the negative. You will need to take the negative log of 0.1 to find the poh.
Therefore, 3.0x10¯³ m naoh will also produce 3.0x10¯³ m oh¯. H two before we can calculate the ph by subtracting the p. You will need to know the molarity of the naoh.
Arrhenius acid act as a good electrolyte as it dissociates to its respective ions in. Sonra, ph + poh = 14 formülünü uygulayın. The relationship between the ph and the p.
There are a few different formulas you can use to calculate poh, the hydroxide ion concentration, or the ph (if you know poh): To calculate the molar concentration of , we can use the known values for the moles of and the volume of solution: Egzersiz yapın [0.1] = 1.
Let's assume the solution is 0.1m. Ph plus ph equals 14. Chemical equilibrium and ionic equilibrium are two major concepts in chemistry.
Of naoh is 0.5 , from this you can calculate poh. Naoh is a strong base, so this will produce 0.1mol/l of oh ions in solution. Poh value of 2×10 −4 m naoh solution is :
Ionic equilibrium deals with the equilibrium involved in an ionization process while chemical equilibrium deals with the equilibrium during a chemical change. (a) 0.000259mhclo4 (b) 0.21 m naoh (c) 0.000071m ba (oh) 2 (d) 2.5m koh. H is found through the number of 14.
So if we know the p o. This will work out to be 1. The poh of naoh is 2.
Three moeller before our ph will be negative. Of naoh is 0.5 , from this you can calculate poh. The concentration of in the solution is.
The poh of a 0.260 m solution of naoh is. A 3.7 b 2.7 c 4.3 d 3.3 medium solution verified by toppr correct option is a) poh =log (2×10 −4) =[log 2+log 10 −4] =−log 2+4 =4−0.3010. Finally, we shall determine the poh of the solution.
Calculate the molar concentration of. That will give you the log of the concentration and then changing the sign by hitting the plus or minus sign and you'll get 4.0. Now you apply poh+ph =14 (at 25 degree centigrade) to calculate ph of 0.5 m naoh.
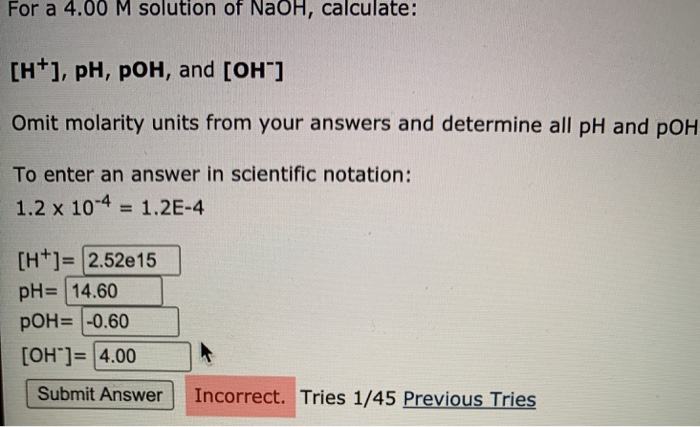
Solved For A 4.00 M Solution Of Naoh, Calculate: [+], Ph, | Chegg.com
